Abstract
Areas of gingival recession cause either an esthetic problem and or root sensitivity. Obtaining predictable root coverage has become an important part of periodontal therapy. A deep, long – standing recession promoted by ANUG was treated using subepithelial connective tissue graft technique combined with decontamination of root surface by using Er:YAG laser. Our clinical findigs suggest that this technique is a predictable procedure to treat gingival recession and promote root decontamination.
Author Contributions
Academic Editor: Gaetano Isola, University of Messina, Italy.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 José Ricardo Kina, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The problem of gingival recession has been treated with a variety of techniques depending upon whether the recession was generalized or on an isolated tooth. The free gingival autograft has been used to cover denuded roots associated with localized gingival recession since the first report on this application of the technique was published by Sullivan and Atkins in9. Langer and Langer5 in describe the use of subepithelial connective graft as a donor source of root coverage specifically for the wide multiple recessions. They advocate success of these grafts to the double-blood supply at the recipient site from the underlying connective tissue base and the overlying recipient flap. However reconstructive gingival therapy requires a carefully root surface treatment by using physical and/or chemical methods. Yukna11 and Miller8 advocate that the graft procedure be preceded by citric acid application to the affected root by periodontal disease. His recommendation for chemical methods is based on reports suggesting that demineralization of root surfaces with citric acid may promote decontamination of root that stimulate cementogenesis. Terranova et al,10 have noted root surface conditioning with tetracycline resulted in an increased number of binding sites for fibronectin that may increase root coverage. The present clinical trial was designed to evaluate the potential of Er-YAG laser to treat root surface previously a root coverage by using subepithelial connective tissue graft technique.
Materials and Methods
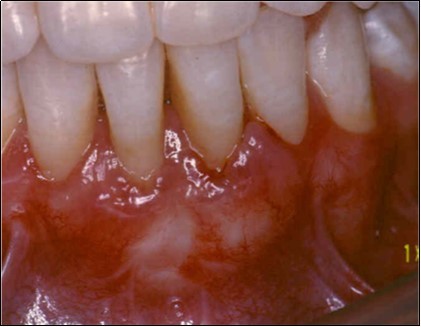
The patient under hard emotional stress that associated with bacterial plaque promoted ANUG with necrosis of alveolar bone and marginal gingival soft tissue in 31 and 41 (Figure 1). The patient was free of systemic disease and was not currently receiving any medication. Initial therapy was carried out using Er-YAG laser, to promote scaling and root planning and oral hygiene instruction. After initial therapy (Figure 2) the surgical method employed consists of the following steps as described by Langer and Langer5.
Figure 2.After initial therapy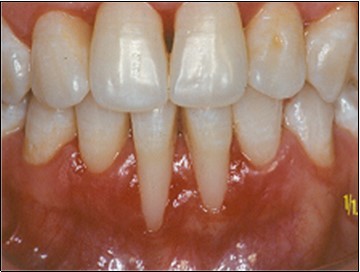
Recipient Site
A partial thickness flap is created with two vertical incisions placed at least one-half to one tooth wider mesiodistally than the area of gingival recession. The coronal margin of the flap is started with a horizontal sulcular incision to preserve all existing radicular gingiva. The interproximal papillae are left intact. The flap dissection is partial thickness leaving connective tissue over the existing bone and / or root surfaces. The root of the involved tooth is carefully scaled and planed until its surface is smooth and hard. Er-YAG laser is then applied to its surface to promote root biomodification, at powers ranging from 25 to 50 mJ/pulse/sec. (Figure 3, Figure 3A).
Figure 3A.Er-Yag laser being applied to promote root biomodification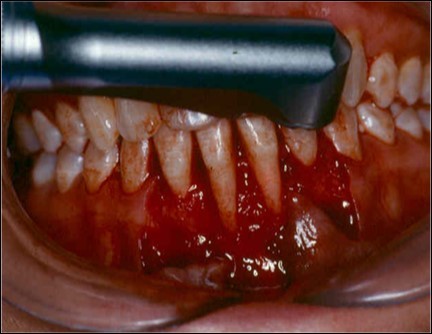
Donor Site
A second surgical site is created on the palate. The length of this is determined by the combines width of the teeth to be covered. The incisions are made in an anterior posterior direction. They are carried to the depth of the palatal mucosa, where they converge just short of the bone. A wedge of tissue is removed as free gingival graft, and its band of epithelium is excised by using Er:YAG laser and acute dissection with scalpel and blade 15C (Figure 4, Figure 4A). The graft is placed in the previously recipient site so that it completely covers the formerly exposed root area (Figure 5). It is then firmly sutured in place with 5-0 gut. The partial thickness, flap is positioned coronally in a manner to cover as much of the graft as possible and sutured in this position to promote maximum adaptation to ensure better healing with less chance of wound dehiscence (Figure 6). The donor site and recipient site is covered with surgical dressing and the patient is instructed in normal postsurgical management. The patient is seen on the 7th postoperative day to remove the surgical dressing and sutures. No additional dressing is necessary, and normal plaque control techniques are resumed (Figure 7)
Figure 4.Donor site area in the palate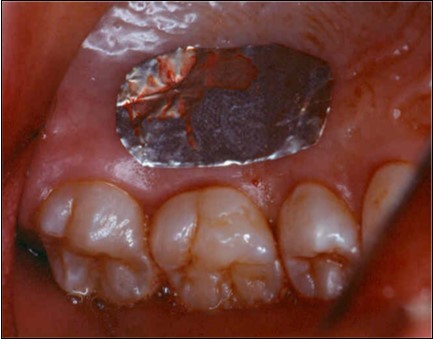
Figure 4a.Elimination of the epithelium from free gingival graft by acute dissection
Figure 5.Subepithelial graft sutured over exposed roots surfaces
Figure 6.The partial thickness flap sutured over subepithelial graft
Discussion
The use of the subepithelial connective tissue graft offers a combination of both the pedicle flap and the free gingival graft. The pedicle flap allows for possible root coverage since it retains its apical blood supply and therefore survives over an avascular root surface5. The free gingival graft supplies a resilient type of connective tissue with a genetic predisposition which ensures thickness and keratinization (Karring and Löe4. The double-blood supply, that from the underlying periosteum and the overlying flap, seems to be enough to nourish the entire graft5. Regarding the effect of the Er:YAG laser for periodontal treatment, its has been advocated because of its potential to decontaminate the diseased root surface and the periodontal pocket7. It may also help to remove the adjacent pocket epithelium and the calculus from root surfaces6,3. Cobbe et al2 and Ando et al1, examined in vivo bactericidal effect and conclude that the exposure of the root surface to laser could significantly decrease the levels of subgingival Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia. In this study the Er:YAG laser was applied at powers ranging from 25 to 100 mJ/ pulse / sec. The laser irradiation was performed under water irrigation, with the tip held perpendicular to the exposed root to promote decontamination of root surface and try to remove the smear layer produced by instrumentation. The laser was also applied on the free gingival graft to remove keratinized epithelium from connective tissue graft. Those procedures seem to be efficient, although histology is not available at this time. However ours clinical results after 2 years have maintained a stable position (Figure 8).
References
- 1.ANDO Y, AOKI A, WATANABE H, ISHIKAWA I. (1996) Bactericidal effect of erbium YAG laser on periodontal bacteria. Lasers Sug. Med., v.19 190-200.
- 2.C M, T K MCcAWLEY, W J KILLOY. (1992) A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. , J Periodontol 63, 701-707.
- 3.ITO K, NISHIKATA J, MURAI S. (1993) Effects of Nd:YAG laser radiation on removal of a surface smear layer root planing: A scanning electron microscopic study. , J Periodontol 64, 547-552.
- 4.KARRING T, N P LA, LÖE H. (1975) The role of gingival connective tissue in determining epithelial differentiation. , J Periodont Res 10, 1.
- 5.LANGER B, LANGER L. (1985) Subepithelial connective tissue graft techique for root coverage. , J. Periodontol., v 56, 715-720.
- 6.R M LUCAS, S Y CHEN, J. (1979) Histochenical study of strain L fibroblasts exposed to endotoxin, The effect on cellular organelles. , J. Periodontal 50, 20-22.
- 8.MILLER P D Jr. (1982) Root coverage using a free soft tissue autograft following citric acid aplication. , Part I: Technique. Int. J. Periodont 2, 65.
- 9.H C SULLIVAN, J H ATKINS. (1968) Free autogenous gingival grafts. III. Utilization of grafts in the treatment of gingival recession. , Periodontics 6, 152.