Abstract
Patients with inflammatory bowel disease (IBD) frequently visit the emergency department (ED). The use of cputed tomography (CT) scans in this population has drastically increased in recent years and may confer an increased risk of malignancy. Records were obtained for IBD patients aged 18 or older who visited our institutional ED with a gastrointestinal chief complaint and who had a CT scan ordered by an ED physician. A predictive model for identifying a clinically actionable finding (CAF) on CT scan was created using logistic regression carried out on a predetermined set of variables. Data were available on 156 Crohn’s disease (CD) patients contributing 350 visits and 63 ulcerative colitis (UC) patients contributing 114 total visits. CAF was identified at 108/350 (30.9%) of visits in CD patients and 33/114 (29.0%) of visits in UC patients. History of CAF (OR 11.6, CI 4.54-29.6) and a platelet count above 400,000/mL (OR 3.42, CI 1.56-7.50) were the strongest predictors of CAF. History of psychiatric illness (OR 0.67, CI 0.35-1.29) and diarrhea (OR .043, CI 0.23-0.83) were associated with a lower likelihood of CAF. A prediction model was created that was able to detect 94.4% of CAF cases while correctly predicting CAF non-cases 35% of the time. This model holds promise as a tool to reduce imaging in this population.
Author Contributions
Academic Editor: Rongbiao Tang, Department of Radiology, Ruijin hospital, School of Medicine, Shanghai Jiao Tong University (SJTU), China
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Michael Loudin, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Inflammatory bowel disease (IBD) affects an estimated 1.5 million Americans.(1) Young age at onset and a relapsing-remitting disease course may lead to frequent and ongoing healthcare system interactions, a substantial proportion of which are in the emergency department (ED). IBD patients made an estimated 76,000 visits to the ED in 2005, an increase of 165% from the previous decade.(2) Abdominopelvic computed tomography (APCT) use in the ED increased in Crohn’s disease (CD) patients from 47% in 2001 to 78% of all ED visits in 2009.(3)
APCTs commonly reveal findings (abscess, perforation, obstruction, et cetera) in this population that change clinical management. Previous studies have shown that 16.8-48% of CD and 12.8% of ulcerative colitis (UC) patients receiving an APCT in the ED had a finding that changed clinical management.(3-9) However, APCTs are associated with a cost and may be associated with an increased risk for malignancy. It is estimated that one APCT confers an increased risk of malignancy of about 6/10,000, which when applied on a population level is estimated to account for 1.5-2% of all cancers in the United States.(10) Additionally, several studies have identified a subgroup of patients with IBD that are high utilizers of imaging studies; these patients may be at a higher risk for harm from the associated radiation than the general IBD population.(11-14) Risk factors associated with increased radiation exposure included young age at diagnosis, prior surgery, and more severe disease.(13) Furthermore, a study examining outpatient use of CT scans by patients with Crohn’s disease identified chronic pain, any psychiatric diagnoses, and frequent missed outpatient appointments as risk factors for high utilization of CT scans.(14)
While prior studies have associated psychosocial variables with a higher utilization of healthcare, no studies have examined whether these variables affect the likelihood of identifying a clinically actionable finding (CAF) on APCT scan in IBD patients presenting to the ED. The aim of this retrospective cohort study was to identify demographic and clinical characteristics that may predict whether a patient with IBD presenting to the ED with a gastrointestinal chief complaint has a CAF on an APCT scan. Furthermore we constructed a model for patients with Crohn’s disease to assist clinical decision-making surrounding the choice to obtain an APCT in this potentially vulnerable population.
Materials and Methods
Prior to initiation of research approval was obtained from the Oregon Health & Science University institutional review board. The Oregon Clinical and Translational Research Institute (OCTRI) cohort discovery tool was used to identify patients at OHSU with a diagnosis of CD or UC by Institutional Classification of Diseases-9th Revision (ICD-9) code 555.x or 556.x (confirmed by review of the medical chart using clinical, endoscopic, histologic, and radiologic findings) with visits to the OHSU emergency department between January 1, 2008 and August 1, 2014 with an APCT ordered during that encounter.
Electronic medical records were manually reviewed (ML, KJ, JL) to determine whether the patients met inclusion criteria and to abstract data. Inclusion criteria were patient age greater than or equal to 18, an established diagnosis of CD or UC at presentation to the ED, a gastrointestinal chief complaint, and an APCT scan ordered by an ED physician (Supplementary Figure 1). Reviewers abstracted a pre-determined set of variables and compared a set of 50 patient encounters to standardize data collection methods. Variables to be abstracted were selected a priori based on the clinical experience of investigators, review of previously published reports on the subject, as well as studies of variables associated with increased use of healthcare by IBD patients. Variables identified included demographic information, clinical and treatment, presenting symptoms, initial laboratory values obtained within 24 hours of presentation, vital, abnormal abdominal x-ray, and medication history.
The OCTRI cohort discovery tool was also used to identify 50 CD patients with ICD-9 code 555.x or 556.x who visited the OHSU emergency department between January 1, 2008 and April 1, 2014 who did not have an APCT ordered during that encounter. These patients met identical inclusion criteria to the expmental group but did not have an APCT ordered by an ED physician and were used as controls. Within this control group a surrogate marker of CAF was used since there were no imaging findings available at their ED visit by which a CAF could be identified. Encounters were examined for CAF diagnosed clinically or via imaging (not ordered by an ED physician) within the associated clinical encounter, or within the 4 subsequent weeks if the patient was discharged from the ED.
APCTs with and without intravenous and oral contrast administration were included. Summarized APCT scan results were reviewed to assess for the primary outcome: a CAF. A CAF was defined as one or more of the following identified on imaging: obstruction, abscess, perforation, acute cholecystitis, cholangitis, appendicitis, diverticulitis, ischemia, vascular emergency, pyelonephritis, renal obstruction, pancreatitis, new neoplasm, complicated urolithiasis, new fistula requiring intervention, or gynecologic emergency. Obstruction was defined by the presence of a transition point on imaging.
Demographic and clinical characteristics measured at the initial ED encounter were examined in CD and UC patients and were compared between patients who did and did not have a CAF identified on APCT in each group in a univariate analysis (Table 1,Table 2). The distributions of continuous-valued characteristics were compared using the Kruskal–Wallis test. Categorical-valued characteristics were compared using the chi-square test or Fisher’s exact test as appropriate.
Table 1. Demographic and clinical characteristics of Crohn's disease patients with univariate analysis, only statistically significant variables displayed. Results are given as percent (n) or median (interquartile range), as appropriate. P-values reflect result of chi-square or Fisher's exact test, as appropriate, for categorical-valued variables, and Kruskal-Wallis test for continuous-valued variables. *Fisher's exact test. CD – Crohn’s disease; CAF – clinically actionable finding; APCT – abdominopelvic computed tomography.| All patients, first visit (N=156) | CAF at first visit (N=67) | No CAF at first visit (N=89) | p-value | |
| Neutrophils | 76 (65,85) | 80 (70,88) | 72 (64,83) | 0.007 |
| Lactate | 1.1 (0.7,3.0) | 3.1 (1.35,3.65) | 0.75 (0.6,1.1) | 0.002 |
| Diarrhea | 36.5% (57) | 22.4% (15) | 47.2% (42) | 0.001 |
| Gastrointestinal bleed | 27.6% (43) | 19.4% (13) | 33.7% (30) | 0.048 |
| Constipation | 11.5% (18) | 17.9% (12) | 6.7% (6) | 0.031 |
| History of obstruction | 48.7% (76) | 73.1% (49) | 30.3% (27) | <0.001 |
| History of abscess | 32.7% (51) | 53.7% (36) | 16.9% (15) | <0.001 |
| History of CD-related surgery | 67.9% (106) | 79.1% (53) | 59.6% (53) | 0.01 |
| Disease phenotype | ||||
| Inflammatory | 36.5% (57) | 22.4% (15) | 47.2% (42) | 0.002 |
| Stricturing | 32.7% (51) | 34.3% (23) | 31.5% (28) | |
| Perforating/Fistulizing | 30.8% (48) | 43.3% (29) | 21.3% (19) | |
| Admission | 76.3% (119) | 91.0% (61) | 65.2% (58) | <0.001 |
| Variable | All patients, first visit (N=63) | CAF at first visit (N=19) | No CAF at first visit (N=44) | p-value |
|---|---|---|---|---|
| Mean outpatient encounters/month | 0.19 (0.02,0.42) | 0.30 (0.14,0.52) | 0.12 (0.01,0.38) | 0.05 |
| APCT in last 30 days | 7.9% (5) | 26.3% (5) | 0.0% (0) | 0.002* |
| APCT in last 6 months | 14.3% (9) | 31.6% (6) | 6.8% (3) | 0.017* |
| Diarrhea | 44.4% (28) | 21.1% (4) | 54.5% (24) | 0.014 |
| Gastrointestinal bleed | 42.9% (27) | 15.8% (3) | 54.5% (24) | 0.004 |
| Constipation | 7.9% (5) | 21.1% (4) | 2.3% (1) | 0.026* |
To identify characteristics that may predict whether CD patients presenting to the ED may have a CAF at a given ED visit a random-effects logistic regression model was built using the available patient-level and visit-level information. A set of easily obtainable, objectively measurable candidate predictors was chosen to include in the model construction based on literature review and a priori clinical considerations: demographic characteristics (age and sex); clinical and treatment history (disease phenotype, CD-related surgery, previous APCTs, and history of obstruction, perianal disease, abscess, or perforation); vital signs (temperature, pulse rate, systolic blood pressure), and laboratory values (platelet count, leukocyte count, albumin). Additionally, history of psychiatric diagnosis including depression, anxiety, schizoaffective disorder, schizophrenia, substance abuse disorder and use of psychiatric medication were included in an effort to amplify the ability of the model to distinguish ED visits that may have been a result of an interaction between CD and an underlying psychiatric condition.
A small number of variables that were missing 80% or more of the values were excluded from consideration in the final model. Multiple imputation was employed to fill in missing covariate values so that visits with incomplete information could be included in analyses. Specifically a sequential imputation using chained equations employing predictive mean matching over the output from a k-nearest neighbor matching algorithm (with k=5) was used.(15, 16) We compared estimates from the complete-case analysis to those obtained from combining results from 500 imputed datasets using Rubin’s rules for coefficient and variance adjustment.(17) The model was internally validated by examining and comparing estimated coefficients when patients were removed from the dataset one at a time. Using these methods a parsimonious model was built by choosing the variables that had the highest impact on the partial area under the curve as a marker of predictive value. History of psychiatric diagnosis was kept in the model regardless of the strength of association to account for the contribution of psychiatric illness to ED visits and to evaluate its impact.
As a sensitivity analysis each of the variables included in the model was removed one at a time and the performance of each of these models was evaluated and compared (Supplementary Figure 2). Additionally, to evaluate whether the model was robust to the potential selection bias introduced by the fact that all patients underwent APCT the performance of the prediction model was evaluated on a set of 50 controls who did not undergo APCT.
Results
Of the 328 patients identified using the OCTRI cohort discovery tool, 219 met inclusion criteria. 156 patients had a diagnosis of CD and accounted for a total of 350 ED visits. 96 (61.5%) CD patients underwent 1 APCT, 43 (27.6%) underwent 2 to 4 APCTs, and 17 (10.9%) underwent 5 or more APCTs. The mean age (±SD) of CD patients at presentation to the ED was 37.9 (±13.3), and 188 (53.7%) of encounters were made by women. One or more CAF was identified on APCT in 108/350 (30.9%) of all CD patient encounters.
63 patients with UC were identified with a total of 114 ED visits. In the UC group 40 patients (63.5%) underwent 1 APCT, 20 (31.7%) underwent 2 to 4 APCTs, and 3 (4.8%) underwent 5 or more APCTs. The mean age (±SD) of UC patients at presentation to the ED was 45.1 (±15.9), and 64 (56.1%) of encounters were made by women. One or more CAF was identified in 33/114 (28.9%) of all UC patient encounters. Supplementary Table 1 and Table 2 identify imaging findings by disease type. (SupplementaryTables 1-2)
Univariate analysis was performed comparing the baseline characteristics of initial patient ED encounters where CAF was identified to those where no CAF was identified. In the CD cohort the group with CAF identified at the first visit had a greater chance of a previous history of obstruction or abscess, prior CD-related surgery, perforating/fistulizing disease, and admission. The CAF group more often presented with constipation, elevated neutrophil count, and elevated lactate, but constipation was uncommon in each group and lab values were missing for many patients. (Table 1) The group with CAF at the first visit had fewer overall APCT scans and were less likely to have diarrhea as a presenting symptom or have the inflammatory phenotype of CD. (Table 1) In the UC cohort the group with CAF identified at the first visit had a greater chance of having an APCT obtained in the preceding 30 days or 6 months were more likely to present with constipation or an abnormal abdominal x-ray, and had an increased number of outpatient encounters per month. (Table 2) Patients with CAF at the first visit were less likely to present with diarrhea or a gastrointestinal bleed. (Table 2)
Predictors for the model were selected by identifying the variables that had the highest impact on prediction of CAF in CD patients. Variables included in the final model were age, history of psychiatric diagnosis, history of CAF, diarrhea, and platelet count > 400,000/mL. (Table 3) When using a probability cutoff of 10%, the performance characteristics of our model were: sensitivity of 94.4% (CI 88.3-97.9%), specificity of 38.4% (CI 32.3-44.9%), positive predictive value of 40.6% (CI 34.5-47.0%), and negative predictive value of 93.9% (CI 87.3-97.7%), and area under the receiver operating curve (AUROC) of 0.7987. (Figure 1) Of the 108 visits where a CAF was identified our model did not predict a CAF in 6 (5.6%). There was no clear pattern in the type of finding in these patients: APCT revealed obstruction (two patients), abscess (two patients), new fistula and intussusception. If this model had been implemented in this set of patients at the 10% probability cutoff, 99 of the 350 visits (93.9%) would have been flagged as unnecessary, consisting of 6 visits where CAF was identified and 93 visits where no CAF was identified. The model maintained good performance in distinguishing between control patients with and without CAF, with an AUROC of 0.7829. (Figure 1)
Figure 1.Area under the receiver operating curve for predictive model (odds scale) applied to a Crohn’s disease patient population who underwent an abdominopelvic computed tomography scan and a control population who did not.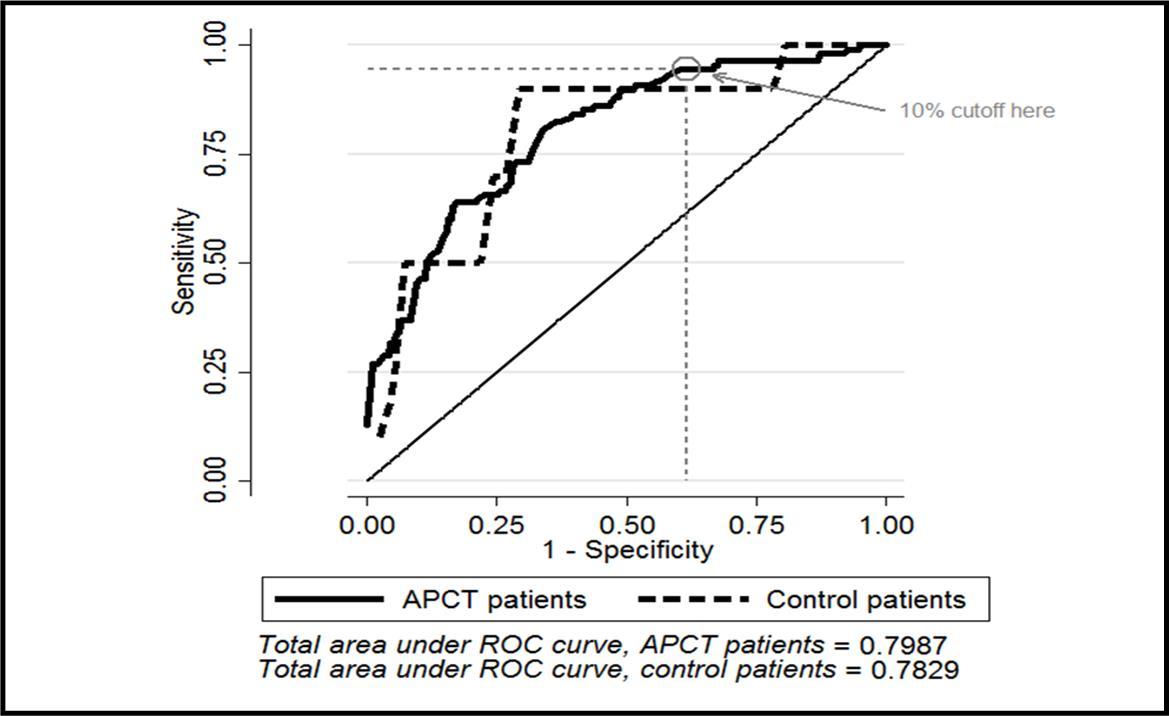
Figure 2.Area under the receiver operating curve for odds scale model, log-odds (3-digit) and single digit models.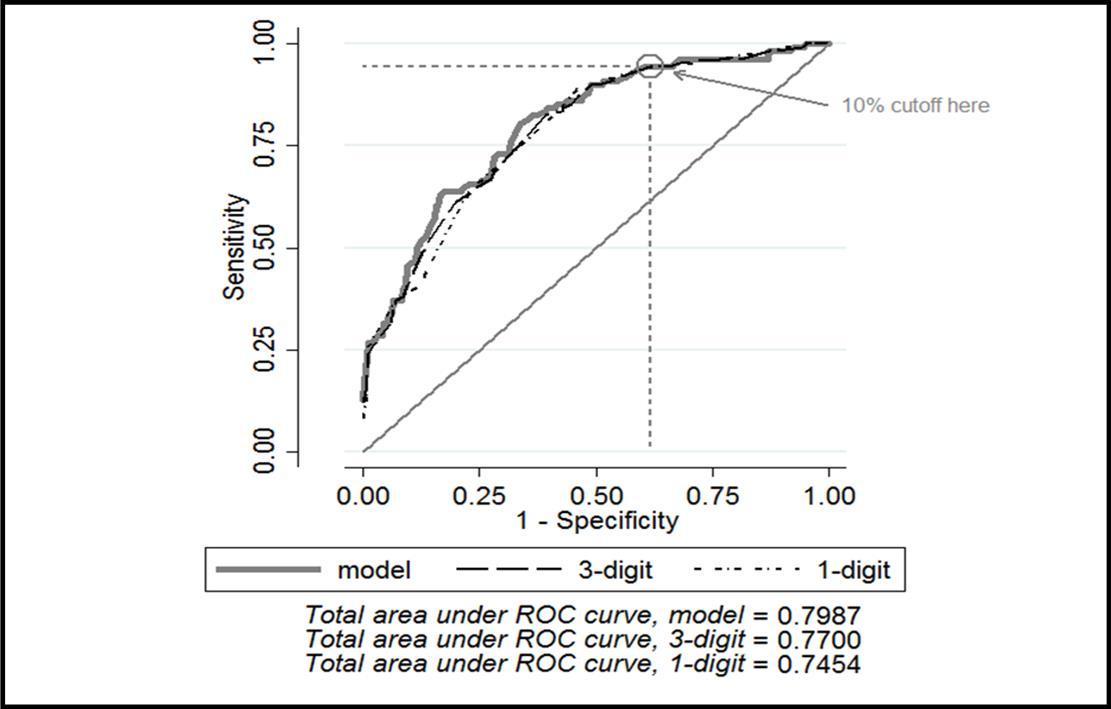
| Clinical Characteristic * | Estimated Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Psychiatric diagnosis | 0.67 | 0.35, 1.29 |
| History of clinically actionable finding | 11.6 | 4.54, 29.6 |
| Diarrhea | 0.43 | 0.23, 0.83 |
| Platelet count ≥ 400,000 | 3.42 | 1.56, 7.50 |
In an effort to simplify the model to allow it to be more easily applied in clinical practice, it was converted from an odds scale (multiplicative) to a log-odds scale (additive). The log-odds scale was further simplified by re-centering at zero and rounding to the nearest integer. (Supplementary Table 3) This streamlining of the model had minimal impact on the AUROC: the original model had an AUROC of 0.7987 and the simplified model 0.7454. (Figure 2) The final, simplified model as it would be applied clinically is portrayed in (Table 4).
Table 4. Final, simplified model (log-odds scale, 1-digit) for identifying Crohn’s disease patients with a low likelihood of having a clinically actionable finding on abdominopelvic computed tomography scan, as would be applied in a clinical setting.| CLINICALLY ACTIONABLE FINDING (CAF) RISK SCORE CALCULATOR |
|---|
| Start at 0 and add or subtract the indicated amount if the specified condition is met:Prior history of CAF?+6Platelet count >400,000+3 (If unobserved, assume a high count)Active diarrhea?-2Prior history of psychiatric diagnosis?-1 (If unknown, assume no history)Age correctionAge 18-45? 0Age 46-55?+1Age 56-65?+2Age 66-75?+5Age 76 or older?+9If the total risk score is:Negative or 0 DO NOT SCANPositive SCAN |
An exploratory analysis was carried out comparing the 17/156 (10.9%) of CD patients who underwent 5 or more APCT scans (a radiation dose that is commonly considered hazardous) during the study period to those who did not.(12, 18) The high-utilizers were much less likely to have a CAF identified at any given visit with 27/145 (18.6%) of APCT scans revealing a CAF in the high-utilizer group compared to 81/205 (39.5%) of APCTs with a CAF identified in those patients who underwent 4 or fewer APCT scans. However, visits by the high utilizers seemed to cluster temporally, with 43% of all visits made by high utilizers occurring at the same age (in years) as the previous visit, compared to only 13% of visits by non-high utilizers. Hence, the lower rate of CAF incidence may be at least partially explained by autocorrelation between visits. Additionally there was little indication that high utilizers and non-high utilizers differed in indicators of acute illness such as vital signs or laboratory values at visits.
Discussion
This study confirms previously published high rates of CAF identified on APCT in patients with CD presenting to the ED with a gastrointestinal chief complaint with one or more CAF identified on APCT in 30.9% of patient encounters.(3-8) Variables most predictive of CAF in CD patients were increasing age, history of psychiatric diagnosis, history of CAF, diarrhea and a platelet count of more than 400,000.
Interestingly our study found a higher rate of CAF in visits by UC patients (28.9%) than a previous study that reported a rate of 12.8%.(7) Examining the rates of individual types of CAF between our two studies the increased rate of CAFs in our study is almost entirely due to a higher incidence of obstruction (10.5% vs 1.9%) and abscess (8.8% vs 2.0%). While inclusion criteria were similar in the two studies, diagnosis of UC was established via established diagnosis of UC at presentation to the ED in both studies, and any given individual had a diagnosis of UC of varying certainty. While UC is not commonly associated with obstruction as a complication previously undiagnosed CD may present this way. Unfortunately we do not have data regarding prior abdominal surgeries such as colectomy in this population, which may also predispose to obstruction. The higher rates of immunomodulatory use, which predispose to infections such as abscess, may help explain the higher incidence of abscesses identified in our patient population compared to the previously reported study.
Prior studies have identified variables associated with increased radiation exposure and high utilization of health care in IBD patients, but none has examined a potential association of psychosocial variables with the probability of identifying a CAF.(11-14) Our study sought to identify whether psychosocial variables may lead to a consistent under reporting or over reporting of symptoms and impact the likelihood that a truly CAF would be identified on imaging. While there were no remarkable differences in these characteristics on univariate analysis between the CD patients who had CAF identified at the first visit and those who did not, a history of psychiatric diagnosis was found to be protective against having a CAF identified on APCT in our predictive model.
We identified a sub-group of 17/156 (10.9%) of CD and 3/63 (4.8%) of UC patients that underwent 5 or more APCT scans ordered by an ED physician, a level of radiation exposure considered potentially harmful on an individual scale.(12, 18) CD patients in our study who underwent 5 or more APCTs were less likely to have a CAF identified on any given scan: 18.6% compared to 39.5% in the patients who underwent 4 or fewer APCTs. Although a higher degree of correlation among visits in the former group may partially explain the contrast, this group may also represent a particularly vulnerable population who present to the ED and are scanned more frequently but are less likely to benefit from each scan.
We also assessed the total number of APCT scans obtained at our institution in any context for these patients and found that 56/156 (35.9%) of CD and 15/63 (23.8%) of UC patients underwent 5 or more APCT scans obtained in any clinical context within the approximately 6-year timeframe of our study. These rates of exposure to radiation from CT scans are higher than those previously reported in the literature with a recent meta-analysis examining ionizing radiation dose across all clinical contexts showed a harmful level of radiation in 11.1% and 2% of Crohn's disease and ulcerative colitis patients respectively.(18) Additionally our data do not account for APCT scans obtained at institutions other than our own and do not account for other forms of ionizing radiation and so could underestimate the true rates of radiation exposure in these patients.
The retrospective and single-center nature of our study imposes inherent limitations. Patients did not experience a standardized workup. Certain variables that may have been of use in a predictive model, such as erythrocyte sedimentation rate, C-reactive protein, lactate, and abdominal plain films, were not obtained in the majority of ED visits. We constructed our model to emphasize sensitivity over specificity as missing a CAF could have severe consequences for a patient and any model that misses a significant amount of CAFs would not be of real world utility. This resulted in a reduction of the number of patients that may be saved an APCT scan based on the predictive model, limiting its utility as a cost- and radiation-reduction tool.
The choice to examine outcomes of APCT scans introduces bias into our study as well as the criteria which ED physicians employ to decide to obtain an APCT scan are uncertain and may vary across patients and practitioners. Finally, the multiplicity of potential CAFs that can be identified on an APCT introduces difficulty in creating a unifying model that may predict any of these complications, a potential explanation for the low specificity of the model.
Our model has proven to be robust. Multiple imputation was used for encounters missing variables but the performance characteristics of the model held up even when using only encounters with complete data sets. Additionally, the model was internally validated by removing patients one at a time, and held up when applied to a control group who did not have an APCT obtained at their ED visit; this suggests that we did not overfit to this one dataset. Sensitivity analysis, performed by removing variables from the predictive model and assessing the impact on performance, revealed that the model was robust to specification error as long as history of CAF (the most influential predictor) was maintained in the model. Finally, to our knowledge we are the first to attempt to associate psychosocial variables with the likelihood of identifying a CAF on imaging.
Additional studies are needed to identify what factors predispose some patients to repeated CAFs. Additionally, further characterization of the subgroup of patients that are high utilizers of CT scans may provide information helpful in identifying and caring for this potentially more vulnerable population. We plan to carry out a prospective validation of this model in our institutional ED.
Conclusion
In summary, the yield of APCT for identifying a CAF was 30.9% in CD patients and 28.9% in UC patients. Our model performed well when used to identify patients with a low likelihood of having a CAF identified on APCT scan, a finding that may aid ED physicians by identifying those patients least likely to benefit from APCT. While our model was internally validated and performed well when applied to a control group, it requires external validation on a larger set of patients.
Acknowledgements
Departmental funding from the Division of Gastroenterology and Hepatology at Oregon Health & Science University was obtained for assistance with statistical analysis.
References
- 1.Ananthakrishnan A N. (2015) Epidemiology and risk factors for IBD. , Nature reviews Gastroenterology & hepatology; 12(4), 205-17.
- 2.Ananthakrishnan A N, McGinley E L, Saeian K. (2010) Trends in ambulatory and emergency room visits for inflammatory bowel diseases in the United States,1994-2005. The American journal of gastroenterology;105(2):. 363-70.
- 3.Kerner C, Carey K, Mills A M. (2012) Use of abdominopelvic computed tomography in emergency departments and rates of urgent diagnoses in Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association;10(1):. 52-7.
- 4.Kerner C, Carey K, Baillie C. (2013) Clinical predictors of urgent findings on abdominopelvic CT in emergency department patients with Crohn's disease. Inflammatory bowel diseases;. 19(6), 1179-85.
- 5.Israeli E, Ying S, Henderson B. (2013) The impact of abdominal computed tomography in a tertiary referral centre emergency department on the management of patients with inflammatory bowel disease. , Alimentarypharmacology,therapeutics; 38(5), 513-21.
- 6.Fishman E K, Wolf E J, Jones B. (1987) CT evaluation of Crohn's disease: effect on patient management. , AJR American journal of roentgenology; 148(3), 537-40.
- 7.Yarur A J, Mandalia A B, Dauer R M. (2014) Predictive factors for clinically actionable computed tomography findings in inflammatory bowel disease patients seen in the emergency department with acute gastrointestinal symptoms. , Journal of Crohn's & colitis; 8(6), 504-12.
- 8.Govani S M, Guentner A S, Waljee A K. (2014) Risk stratification of emergency department patients with Crohn's disease could reduce computed tomography use by nearly half. Clinical gastroenterology and hepatology : the official clinical practice journal of the. , American Gastroenterological Association; 12(10), 1702-7.
- 9.Jung Y S, Park D I, Hong S N.. (2015)Predictors of Urgent Findings on Abdominopelvic CT in Patients with Crohn's Disease Presenting to the Emergency Department. Digestive diseases and sciences; 60(4), 929-35.
- 10.Brenner D J, Hall E J.(2007)Computed tomography--an increasing source of radiation exposure. The New England journal of medicine;. 357(22), 2277-84.
- 11.Kroeker K I, Lam S, Birchall I. (2011) Patients with IBD are exposed to high levels of ionizing radiation through CT scan diagnostic imaging: a five-year study. , Journal of clinical gastroenterology; 45(1), 34-9.
- 12.Newnham E, Hawkes E, Surender A. (2007) Quantifying exposure to diagnostic medical radiation in patients with inflammatory bowel disease: are we contributing to malignancy? Alimentary pharmacology, therapeutics;. 26(7), 1019-24.
- 13.Desmond A N, O'Regan K, Curran C. (2008) Crohn's disease: factors associated with exposure to high levels of diagnostic radiation,Gut;57(11):. 1524-9.
- 14.Young W, Hyman N, Osler T. (2013) Predictors of excessive CT scan use in a surgical cohort of patients with Crohn's disease. Postgraduate medicine;. 125(6), 94-9.
- 15.White I R, Royston P, Wood A M. (2011) Multiple imputation using chained equations: Issues and guidance for practice. Statistics in medicine;. 30(4), 377-99.
Cited by (1)
- 1.Riskin Geuz Kinneret S., Schwartz Doron, 2022, Less Emergency Department Abdominopelvic Computed Tomography for Patients With Crohn’s Disease, Journal of Clinical Gastroenterology, 56(8), 712, 10.1097/MCG.0000000000001634
