Abstract
Successful viral load programs rely on the presence of data systems and high quality of patient data. Using a cohort of 49 patients at Partners in Hope, a large, urban HIV clinic in Malawi, we performed a quality improvement assessment of a new viral load program with a focus on accuracy of data collected from patients as well as adherence to Malawi HIV Guidelines in regard to response to elevated viral loads (≥1,000 copies/mL). Data were obtained from three parallel medical record systems to investigate the proportion of patients with a repeat viral load and whether the three data systems agreed in regard to sociodemographic and clinical data. Fewer than 30% of patients had a repeat viral load within six months, as recommended in the Malawi HIV Guidelines. There were significant problems with data agreement across the three parallel databases used for care. Date of birth was consistent for 55.1% (N=27) of patients, while a different date of birth was noted in all three sources for 10.2% of patients (N=5). Viral load data from all three sources agreed for only 2.0% of patients (N=1). For 65.3% (N=32), the viral load from the laboratory did not match the recorded viral load in the electronic or paper record. Scale-up of viral load monitoring must be accompanied by the development of data systems that support workflow from sample collection to lab and back to provider. Education of providers and strategies for data collection with minimal errors can facilitate scale-up of high quality programs.
Author Contributions
Academic Editor: Dr Shivaji Kashinath Jadhav, Sandor Life Sciences Pvt Ltd/ NIMR,Indian Council of Medical Research, NIMR, Goa
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Jean Gibb, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
In 2016, the World Health Organization (WHO) recommended viral load monitoring as part of routine care in all settings1. Viral load monitoring was introduced in Malawi in 2011, and in the 2016 Malawi HIV Guidelines viral load is recommended as part of routine care, with an initial viral load six months after antiretroviral therapy (ART) initiation, a second viral load 24 months after initiation, and subsequent viral loads every 24 months1, 2. Those with a viral load ≥1,000 copies/mL require adherence counseling and repeat viral load in three months, with switch to second line if viral load remains elevated.
Of the sub-Saharan African nations South Africa is generally accepted to have the most developed healthcare infrastructure. Viral load programs have shown promising results, demonstrating that routine viral load monitoring can be cost-effective3. Extrapolation of this data to Malawi is difficult given drastically different infrastructure, limited equipment, challenging transportation of samples to central laboratories, and lack of systems for results reporting. Previous studies have shown viral load monitoring is feasible in Malawi, although most programs to date have been limited in scope and geographic coverage4, 5.
Successful viral load programs rely on the presence of data systems and appropriate management and quality of patient data6, 7. Successful results reporting of viral load relies on collection of accurate identifying patient information and systems for transferring data from central laboratories to health facilities and patient charts8, 9, 7. We performed a small quality improvement assessment of the existing viral load program at Partners in Hope (PIH), a large, urban HIV clinic in Lilongwe, Malawi. Our focus was on accuracy of data collected from patients at the time of viral load sampling and adherence to Malawi HIV Guidelines with regard to the need for a repeat viral load. Since December 2013, PIH has performed viral load testing for approximately 5,000 patients on ART using an on-site Abbott m2000 RealTime System. Medical record keeping at PIH includes three parallel systems. The oldest is a paper “mastercard,” which includes patient identifiers, ART number, a clinic number, appointment dates, ART regimen, dispense dates, pill counts, and previous viral load results (if performed). The second system is a national ART electronic medical record (EMR) endorsed by the Ministry of Health (also known as Baobab), which recapitulates much of the information on the mastercard in an electronic format. The third system, the laboratory information management system (LIMS), stores patient identifiers and viral load results. Data from LIMS represent the “true” viral load results for patients since these data are generated directly from the Abbott machine at the time the tests are performed.
Our quality improvement initiative had two primary aims: (1) to assess the frequency with which patients with elevated viral loads received appropriate repeat testing, and (2) to assess the quality and consistency of data across the three systems: the mastercard, the National ART EMR, and LIMS. We aimed to use this data to improve the quality of the viral load program at PIH and to disseminate lessons learned to similar programs implementing viral load testing in Malawi and sub-Saharan Africa.
Methods
We identified a cohort of 49 patients at Partners in Hope who had been on ART at least six months with an initial measured viral load of ≥1,000 copies/mL obtained between June 1, 2014 and August 31, 2014. This cohort was selected to be large enough to capture general trends in viral load follow up, and small enough to allow for detailed data collection from the three PIH medical records over a three-week period in August 2015.
Data were collected from the three parallel medical record systems: (1) paper mastercards, (2) the National ART EMR, and (3) LIMS, the viral load machine database. Using a paper-based data collection tool, we collected demographic data and the date and value of the elevated viral load during the interval under evaluation for each patient. Data from each of these medical record sources were compared to assess the consistency of documentation. Any data point that was unavailable or unclear from review of medical records was documented as missing. Although the guidelines recommend a repeat viral load test three months after an initial elevated value, we investigated each of the medical record sources for documentation of a repeat viral load within the 18 months following the initial test in order to capture viral load tests completed later than the timeframe recommended in guidelines, and to characterize the timing of “late” viral loads. We also obtained data on appointment adherence for up to three appointments preceding the visit when the patient had an elevated viral load. The appointment was considered missed if the patient presented for the appointment more than 14 days after the scheduled date. At the time of data collection, we recorded whether patients met the Malawi program default criteria (i.e., without ART for 60 days or more), whether they were deceased, transferred out, or whether they were alive and on ART.
Results
Viral Load Data
Of the 49 records reviewed, 71% of patients were female, and the median age was 32 years. Based on the LIMS data, the median initial viral load was 35,386 copies/mL (IQR: 3,605 copies/mL to 102,463 copies/mL) with 36.7% (N=18) between 1,000-10,000 copies/mL, 38.9% (N=19) between 10,001-100,000 copies/mL, and 24.4% (N=12) >100,000 copies/mL. Figure 1 illustrates the distribution of viral loads across the population sampled. A repeat viral load was obtained for 30.6% (N=15) of patients. A repeat viral load was obtained prior to three months for 4.1% (N=2) of patients and between three and six months for another 4.1% (N=2) of patients. The remainder (22.4%, N=11) were obtained more than six months after the first value. Two patients (4.1%) completed a viral load test “on time” at three months (+/- 14 days), as recommended by the Malawi HIV Guidelines. The median time to repeat viral load across the cohort was 42.3 weeks (IQR: 26.6 – 55.9 weeks).
Figure 1.Depiction of the range of viral load results for the 49 patients reviewed. All patients had a viral load>1,000 copies/mL, the lowest limit of detection used in the Malawi program.
The median repeat viral load for the cohort was 2,333 copies/mL, (IQR: 40 - 376,000 copies/mL). Thirteen percent (N=2) had a repeat viral load that was between 200 and 1,000 copies/mL and 20% (N=3) had a viral load <200 copies/mL. Of the initial 49 patients in our cohort, 77.6% (N=38) were documented as alive and on ART at the time of data collection, and 16.3% (N=8) met default criteria. One patient transferred to another clinic, and one patient was deceased. In the year leading up to the date of elevated viral load, 20.3% (N=10) of patients missed one appointment, 22.4% (N=11) missed two appointments, and 22.4% (N=11) missed three appointments or more.
Data Quality and Agreement
Comparing patient demographic data across all three medical record systems, the date of birth was consistent for 55.1% (N=27) of patients, while a different date of birth was noted in all three sources for 10.2% of patients (N=5). The national ART number was consistent among all three systems for 71.4% (N=35) of patients. For 20.4% of patients (N=10), LIMS had a different ART number than the National ART EMR and the paper mastercard. Viral load data from all three sources agreed for only one patient in the cohort. For 65.3% (N=32) of patients, the viral load from LIMS (the “true” viral load) did not match the viral load in the National ART EMR or on the mastercard. The date of viral load collection recorded in LIMS differed from the other two sources for 69.4% (N=24) of patients. Data showing inconsistency among the three sources are summarized in Figure 2.
Figure 2.Quality and Consistency of Patient Medical Record Data Across Platforms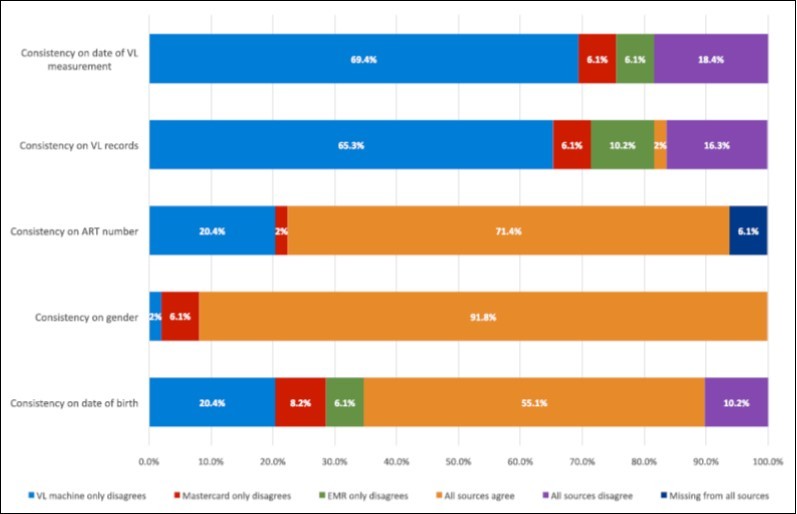
Discussion
In this small group of patients on ART in an urban center with an on-site viral load machine, less than 30% of individuals with a viral load greater than 1,000 copies/mL had a documented repeat viral load within the next year. Of those who did receive a second viral load, only 10.2% were completed within six months and only two were completed at three months as the guidelines recommend. Only 16.3% of patients reviewed in this cohort met default criteria at the time of our data collection, indicating a large proportion of our patients were still in care but had not yet received a repeat viral load. A prolonged delay in a repeat viral load can result in health consequences to patients, including accumulation of resistance mutations in those failing treatment due to resistance, and/or infectious or non-infectious co-morbidities known to be associated with uncontrolled viral replication10, 11.
One reason for low rate of repeat viral load testing may be lack of provider knowledge about guidelines and/or the importance of timely repeat viral load. Educating providers on the benefits of viral load monitoring is an important intervention to accompany viral load scale-up. This education should cover the importance of screening patients and identifying those at high risk for virologic failure as well as recognizing those at low risk to reduce unnecessary monitoring for patients doing well on ART3, 7.
A second reason for the low rate of repeat viral load testing may be difficulty with viral load results being returned to patient files to be utilized by providers for action. The ART number is the identifier by which patients are referenced in the viral load machine itself (LIMS), but this number did not agree with the other data sources for 20.4% of patients. In addition, there were a large number of errors in other data that might be used to link viral load data to a patient when the ART number is incorrect. Manual entry of patient data into the LIMS machine is case sensitive; therefore, a small change such as not capitalizing a letter or capitalizing all letters results in the inability to link LIMS to other data sources or to other viral load results for the same patient within LIMS. For example, a repeat viral load ordered for the patient will not appear when the name “Jane Doe” is entered into a search field if that viral load was entered under “jane doe.” Additionally, in Malawi, the same person may use different names or different spellings of first or last names. Variations of a single letter result in disagreement among the systems and in viral load results being incorrectly linked or not linked at all to the source patient. Birth dates in Malawi are also problematic, as individuals are often unable to provide a specific date. A default date of January 1 is used when patients do not know an exact date of birth. This makes date of birth unreliable as a way to link viral load data or double check a result that may have disagreement in the name and/or ART number.
Our program did not explicitly perform an initial training with providers around the importance of how data for viral load are collected and did not anticipate the need for additional support to ensure accuracy of data entry. Interventions such as orientation of providers to laboratory requisitions for viral loads or implementation of an automated system (i.e., printed stickers) that spares providers from handwriting information onto requisitions could reduce the frequency of errors and improve the accuracy of data entered into the LIMS system and therefore the ability of viral load results to be returned correctly to patient charts. Automated systems, while ideal, often require reliable electricity and back-up electricity (e.g., batteries or generators) and also support for maintenance. While this may be feasible in our large urban clinic, this may not be a solution in rural sites with less infrastructure and support.
Reports on viral load scale-up in southern Africa have revealed challenges in successfully obtaining and acting upon elevated viral loads. A CDC review of viral load scale-up programs cited difficulties in specimen transport, lack of staff, delays in equipment repair, and inadequate laboratory management systems as major challenges to routine viral load implementation12, 13. For PIH, many of these challenges are bypassed by having viral load measurement on site and adequate staffing to operate the LIMS system and viral load machine. Yet, even in this relatively well-resourced clinic, successful viral load scale-up has been difficult due to duplicative data management systems and susceptibility of these systems to error.
Limitations
This project was intended as a small-scale quality improvement initiative in the early phase of the viral load scale-up at Partners in Hope, and as such, we evaluated a small sample, which may not be representative of all patients at this large clinic or at other clinics in Malawi. All clinics in Malawi do not use the same three medical records systems as those utilized by PIH, making lessons learned less generalizable; however, most clinics combine at least two different data collection strategies, thus general principles around data quality and consistency may still apply in these settings. PIH has an on-site laboratory with capacity to measure viral load within the clinic facility. This is atypical in Malawi, and it is likely that viral load scale-up at sites utilizing off-site labs will have additional challenges in regard to data systems and results reporting. Our experience may underestimate the challenges in these settings.
Conclusions
Scale-up of viral load in resource-limited settings must be accompanied by the development of data systems that support the flow of information from the point of sample collection to the central lab and back to the provider and patient with minimal errors on an efficient timeline that provides for clinical information that is relevant and can improve patient outcomes. Education of providers about viral load guidelines, data systems, and strategies for data collection with minimal errors can facilitate scale-up of high quality programs. Viral load monitoring is important to optimize the long-term health of people living with HIV. In order to achieve this goal, viral load programs need to consider all components of the viral load program and empower and educate across this continuum.
Acknowledgements
This research was made possible with support from funding provided by the President's Emergency Plan for AIDS Relief (PEPFAR) through USAID-Malawi under the terms of Grant No. 674-A-00-10-00035-00. We gratefully acknowledge all of the patients and staff at the Partners in Hope Moyo Clinic, where these data were collected. We are thankful to the Lilongwe-based EQUIP-Malawi staff for providing administration and oversight for this project.
References
- 1. (2016) World Health Organization.WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach.
- 3.Phillips A, Shroufi A, Vojnov A, Cohn J, Roberts T. (2015) Sustainable HIV treatment in Africa through viral-load-informed differentiated care. , Nature 528(7580), 68-76.
- 4.S E Rutstein, M C Hosseinipour, Kamwendo D, Soko A, Mkandawire M. (2015) Dried blood spots for viral load monitoring in Malawi: feasible and effective. PLoS One. 10(4), 0124748.
- 5.Maman D, Chilima B, Masiku C, Ayouba A, Masson S. (2016) Closer to90-90-90.The cascade of care after 10 years of ART scale-up in rural Malawi: a population study. , J Int AIDS Soc 19(1), 20673.
- 6.Jobanputra K, Parker L A, Azih C, Okello V, Maphalala G. (2014) Impact and programmatic implications of routine viral load monitoring in Swaziland. , J Acquir Immune Defic Syndr 67(1), 45-51.
- 7.Roberts T, Cohn J, Bonner K, Hargreaves S. (2016) Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clin Infect Dis. 62(8), 1043-1048.
- 8.Dansereau E, Gakidou E, Ng M, Achan J, Burstein R. (2015) . Trends and Determinants of Antiretroviral Therapy Patient Monitoring Practices in Kenya and Uganda. PLoS One 10(8), 0135653.
- 9.Peter T, Ellenberger D, Ki A A, Boeras D, Messele T. (2016) Early antiretroviral therapy initiation: access and equity of viral load testing for HIV treatment monitoring. Lancet Infect Dis.
- 10.Habib O R, Bartlett J A, Thielman N M, Pence B W, Kimani S M. (2016) The Effect of Switching to Second-Line Antiretroviral Therapy on the Risk of Opportunistic Infections Among Patients Infected with Human Immunodeficiency Virus in Northern Tanzania. Open Forum Infectious Diseases.3(1),ofw018.
- 11.Sigaloff K C, Hamers R L, Kityo C, Siwale M, Ive P. (2013) Unnecessary antiretroviral treatment switches and accmulation of HIV resistance mutations; two arguments for viral load monitoring in Africa.J Acq Imm Def Syn.2011;58(1):. 23-31.
Cited by (4)
- 1.Wambura Mwita, Nyato Daniel Josiah, Makyao Neema, Drake Mary, Kuringe Evodius, et al, 2020, Programmatic mapping and size estimation of key populations to inform HIV programming in Tanzania, PLOS ONE, 15(1), e0228618, 10.1371/journal.pone.0228618
- 2.Shroufi Amir, Van Cutsem Gilles, Cambiano Valentina, Bansi-Matharu Loveleen, Duncan Kristal, et al, 2019, Simplifying switch to second-line antiretroviral therapy in sub Saharan Africa, AIDS, 33(10), 1635, 10.1097/QAD.0000000000002234
- 3.Waljee Akbar K, Weinheimer-Haus Eileen M, Abubakar Amina, Ngugi Anthony K, Siwo Geoffrey H, et al, 2022, Artificial intelligence and machine learning for early detection and diagnosis of colorectal cancer in sub-Saharan Africa, Gut, 71(7), 1259, 10.1136/gutjnl-2022-327211
- 4.Nicholas Sarala, Poulet Elisabeth, Wolters Liselotte, Wapling Johanna, Rakesh Ankur, et al, 2019, Point‐of‐care viral load monitoring: outcomes from a decentralized HIV programme in Malawi, Journal of the International AIDS Society, 22(8), 10.1002/jia2.25387
