Abstract
The mitotic count is the most frequent reason for discordance between pathologists in modified Bloom and Richardson (mBR) scoring. Recently, the phosphohistone H3 (PHH3) immunohistochemical stain has been proposed as a potential surrogate marker for mitotic figures. This study examines the differences between H&E mitotic count, PHH3 mitotic count, and Ki-67 index in invasive breast carcinoma. A retrospective review of invasive breast carcinoma cases from 2013- 2014 was performed. H&E and PHH3 mitotic counts were assigned a mitotic score of 1 to 3 using mBR criteria. Ki-67 index was categorized into a three-grade system: <10% (low), 10 - <20% (intermediate), and >20% (high). A total of 451 cases were evaluated. PHH3 versus H&E mitotic count changed mBR scores in 24% of cases, upgrading in 23% and downgrading in 1%. A total of 431 cases had both Ki-67 and PHH3 available for comparison. Both H&E and PHH3 mitotic scores correlated with Ki-67 in 51% of cases; however, PHH3 had better correlation. We conclude that PHH3 in breast carcinoma allows for a more sensitive and practical approach in the identification of mitotic figures. PHH3 IHC is useful as a confirmatory tool in assessing the final mitotic score for more accurate mBR scoring and grading. In this study, 48 out of 451 (10.6%) of patients had a significant upgrade that may change the patient's treatment plans, including the addition of chemotherapy
Author Contributions
Academic Editor: Raffaele Serra, University Magna Graecia of Catanzaro
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Jennifer S. Woo, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
The modified Bloom and Richardson (mBR) scores (also known as Nottingham Histologic Score) and grades play an essential role in the prognosis of breast cancer. They are composed of the three histological parameters: tubule formation, nuclear pleomorphism, and mitotic figures1, 2, 3, 4 Mitotic count difference is the most common reason for discordance in mBR score and grade between pathologists5.A more sensitive marker with greater sensitivity and specificity for mitoses includes antiphosphohistone-H3 (PHH3), which binds more specifically to epitopes of histone H3 that are selectively phosphorylated during mitosis. PHH3 immunohistochemical (IHC) stain analysis is known to have significantly increased interobserver agreement in mitotic rate calculation in recent studies6 In this study, we sought to examine the differences between a routine mitotic count on H&E and PHH3 IHC stained slides. We also analyzed the mBR scores, tumor grades and relation to Ki-67 IHC results to address the final question: does PHH3 IHC aid in providing more accurate analysis of mitotic count which will alter mBR score and tumor grade?
Material and Methods:
Consecutive invasive breast cancer cases from April 2013 to August 2014 were selected in this study after institutional IRB approval. All cases were obtained from breast cancer samples including core needle biopsy, excisional biopsy/lumpectomy, and mastectomy. Neoadjuvant chemotherapy excisional specimens were excluded from the study. Cold ischemic times for core needle biopsy, excisional biopsy/lumpectomy, and mastectomy were recorded. For core needle biopsy specimens, cold ischemic time was less than 1 minute. For all the excisional biopsy specimens, cold ischemic time was less than 1 hour. Clinical information and pathology diagnoses were obtained from a review of the pathology reports. All specimens were fixed in 10% neutral-buffered formalin for 6 to 72 hours as per American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines.
Immunohistochemical (IHC) Analysis:
Immunohistochemical (IHC) analysis was performed on a 4-μm thick section of formalin-fixed, paraffin-embedded tumor tissue. PHH3 IHC analysis was performed using rabbit anti-PHH3 antibody (Abcam, Cambridge, MA) with 1:600 dilution in phosphate-buffered saline. Ki-67 analysis was performed using the M1B1 antibody (Dako, Carpenteria, CA) with 1:100 dilution using the incubation time of 45 minutes with appropriate positive and negative control samples. Slides were baked for 1 to 4 hours in a 60°C oven and then deparaffinized in xylene and brought to tap water through graded alcohols (100% 4 times; 95% 2 times). Slides were then treated with 0.5% hydrogen peroxide in absolute methyl alcohol for 10 minutes to quench endogenous peroxidase activity and later washed with tap water and deionized water. Heat-induced antigen retrieval was then performed by placing slides in 0.01 mol/L citrate buffer, pH 6.0, preheated to 95°C in a vegetable steamer (Black & Decker, Baltimore, MD); heat-treatment lasted for 25 minutes, followed by a 15-minute, room-temperature, cool-down period. The detection system and instrument used were the DAKO (Carpenteria, CA) and Leica-Bond autostainer for Ki-67 and PHH3, respectively.
Microscopic Examination:
H&E mitotic figures, PHH3, and Ki-67-stained slides from each respective case were examined by a total of four breast pathologists (SA, NM, PS, or DL). The field diameter of all four different microscopes used by the breast pathologists microscope was 0.55 mm. Mitotic counts were performed manually on H&E slides at 40x objective (10x eye piece) in 10 consecutive HPF in the most mitotically active areas of the tumor, usually at the tumor periphery. Mitotic figures were defined as cells in recognizable prophase, metaphase, or anaphase and excluded apoptotic bodies. Mitotic scores were assigned based on mBR criteria, accounting for 0.55mm microscope field diameters by H&E slide. Ki-67 and PHH3-stained slides were reported the same day or as addendum on each case the next day (H&E assessment of mitotic rate was independent of IHC stains). The Ki67 and PHH3 stains were assessed as the proportion of tumor cells with positively stained nuclei. Mitotic counts were then performed manually on PHH3-stained slides over 10 HPF similar to the H&E stain method. (Figure 1)Cells in morphologic prophase, metaphase, anaphase, and interphase showing strong dense nuclear staining for PHH3 were considered positive for mitotic figures. (Figure 2) The Ki-67 proliferation index was expressed as the overall percentage of tumor cells with nuclear staining. Overall/average percentage of Ki-67 was recorded looking at the entire tumor fields, including hot spots. Number of Ki-67 positive cells was not counted to obtain Ki-67.
As with H&E mitotic counts, mitotic counts from the PHH3-stained slides were assigned a mitotic score using mBR criteria. Final mBR histologic grades using PHH3 mitotic scores were calculated by adding the mitotic scores to the tubular formation score and the nuclear grade score.
To compare the Ki-67 proliferation index with H&E and PHH3 mitotic scores, the Ki-67 index was categorized into a three-grade system: <10% (low), >10 - <20% (intermediate), and >20% (high).
Figure 1.Images of the tumor cells from the same area (400X). A: H&E stain. B: Ki-67 IHC stain. C: PHH3 IHC stain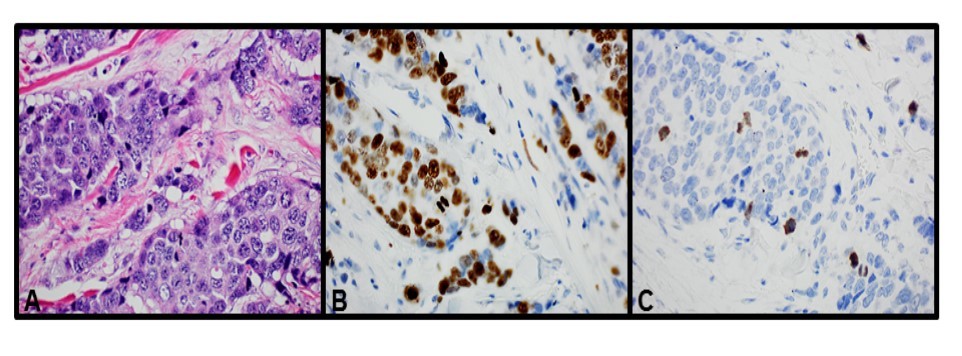
Figure 2.Tumor cells by PHH3 IHC (1000x). A. prophase. B. metaphase. C. anaphase D. Apoptosis, non-specific staining by IHC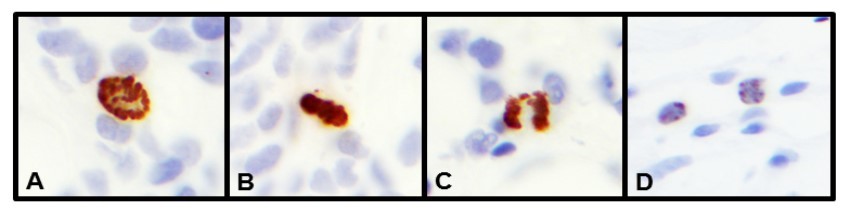
As with H&E mitotic counts, mitotic counts from the PHH3-stained slides were assigned a mitotic score using mBR criteria. Final mBR histologic grades using PHH3 mitotic scores were calculated by adding the mitotic scores to the tubular formation score and the nuclear grade score.
To compare the Ki-67 proliferation index with H&E and PHH3 mitotic scores, the Ki-67 index was categorized into a three-grade system: <10% (low), >10 - <20% (intermediate), and >20% (high).
Results:
A total of 451 cases were evaluated. PHH3 IHC mitotic count changed mBR grades in 24% of the total cases (107/451), upgrading in 23% (101/451) and downgrading in 1% of the cases (6/451) (Figure 3). No change in mBR grade was seen in 76% of total cases (344/451) when using PHH3 versus H&E mitotic count. mBR upgrades were as follows: 53 cases upgraded from grade 1 to grade 2 (12% of total cases), 41 cases upgraded from grade 2 to grade 3 (9% of total cases), and 7 cases upgraded from grade 1 to grade 3 (1.5% of total cases). mBR downgrades from grade 2 to grade 1 was seen in 2 cases (0.5% of total cases) and grade 3 to grade 2 in 4 cases (1% of total cases) (Table 1).
Figure 3.Distribution of cases with mBR grade upgrade and downgrade.
| mBR Grade Change | No. of cases with grade change | % of total cases (n=451) | % of cases with mBR grade change (n=107) | |
| Upgrade | % of cases with mBR grade upgrade (n=101) | |||
| Grade 1 to Grade 2 | 53 | 12 | 49 | 52 |
| Grade 2 to Grade 3 | 41 | 9 | 38 | 41 |
| Grade 1 to Grade 3 | 7 | 1.5 | 7 | 7 |
| Downgrade | % of cases with mBR grade downgrade (n=6) | |||
| Grade 2 to Grade 1 | 2 | 0.5 | 2 | 33 |
| Grade 3 to Grade 2 | 4 | 1 | 4 | 67 |
A total of 431 cases had both Ki-67 and PHH3 IHC stains available for comparison. The Ki-67 proliferation index was concordant with H&E and PHH3 mitotic scores in 51% of cases (220/431). Ki-67 correlated with PHH3 mitotic score alone in 19% of cases (82/431) and correlated with H&E mitotic score alone in 8% of cases (34/431). There was no concordance between Ki-67 and either one of PHH3 or H&E mitotic score in 22% of cases (95/431).(Table 2).
Table 2. Comparison of Ki-67 IHC to H&E and PHH3 IHC.| Ki-67 IHC compared to H&E and PHH3 IHC | No. of cases (% of total cases, n = 431) |
|---|---|
| Concordant Ki-67 and H&E score only | 34 (8%) |
| Discordant Ki-67, H&E, and PHH3 scores | 95 (22%) |
| Concordant Ki-67 and PHH3 score only | 82 (19%) |
| Concordant Ki-67, H&E, and PHH3 scores | 220 (51%) |
Discussion:
The modified Bloom and Richardson (mBR) score and grade (also known as Nottingham Histologic Score) play an essential role in the prognosis of breast cancer. They are composed of the three histological parameters: tubule formation, nuclear pleomorphism, and mitotic figures1, 2, 3, 4Tubular formation is assessed as follows: >75%, 10% to 75%, and <10%, as score 1, 2, and 3, respectively. Nuclear pleomorphism is assessed as mild, moderate, and marked, corresponding to a score of 1, 2, and 3, respectively. The mitotic count score is performed on unambiguous mitoses in 10 consecutive high power (400x) fields in the most cell-dense area in the tumor periphery. Depending on the microscopic field diameter, which determines the mitotic number cut-off values, a score of 1, 2, or 3 is then given. The individual scores of each of the three histologic parameters are then added together. A sum of 3-5 is assigned as grade 1, 6-7 is assigned as grade 2, and 8-9 is assigned as grade 3. A conventional mitotic count on hematoxylin and eosin (H&E) stained slides is recommended since it correlates strongly with clinical outcomes such as progression-free survival. However, mitotic count difference is the most common reason for discordance in mBR scores and grade between pathologists5 Some challenges to standardized reporting of mitotic rate include variable and heterogeneous areas of tumor, the time needed to count and locate the mitoses, and apoptotic bodies mimicking mitoses.
A surrogate marker for proliferation, such as Ki-67, can predict responsiveness to chemotherapy, with proliferative tumors demonstrating increased responsiveness 7. Ki-67 is a proliferative index which not only aids in mitotic rate assessment, but also acts as a powerful prognostic tool since increased Ki-67 has been shown to correlate with shorter cancer survival6, 14 Moreover, adjuvant chemotherapy is significantly beneficial for patients with rapidly proliferating tumors, but not for those with slowly proliferating tumors. For these reasons, immunohistochemistry (IHC) for Ki-67 is performed in addition to other standardized breast biomarkers such as estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu8 However, Ki-67 is not specific for mitotic activity, as it reflects the activity of a wider spectrum of the proliferating cells. Ki-67 expression of cells varies throughout the different cell-cycle phases in G1, S, G2, and M phases, but not during the resting phase G0. Ki-67 expression is low in the G1 and S phases and rises to peak levels in mitosis during interphase, prophase and metaphase. During the late mitotic phase (anaphase and telophase), a sharp decrease in Ki-67 levels occurs9.
It is exceedingly rare to find an H&E mitotic count higher than Ki-67. Ki-67 assessment is based on the percentage of tumor cell nuclei stained by the antibody. However, there is no standard way to assess Ki-67, including how to count positive cells, determine the best cutoff point, decide how many cells to count, and select which areas to read on the slide such as hot spots versus overall tumor cells or combining both areas and giving an average percentage on low power. Since Ki-67 is not specific for mitoses, new surrogate IHC markers for proliferation that are more specific and sensitive to the M phases of the cell cycle are being developed.
The most prevalent markers with greater sensitivity and specificity for mitoses include Antimitotic protein monoclonal-2 (MPM-2), which recognizes phosphoprotein epitopes on mitosis-specific molecules, including topoisomerase IIa, microtubule-associated proteins, Cdc2-inhibitory kinases, and antiphosphohistone-H3 (PHH3). Phosphohistone H3 (PHH3) labeling has been shown to be stronger prognosticators than classical indices such as axillary lymph node status, tumor size, nuclear grade, and histological grade10, 11, 12, 13, 14, 15, 16. Hence, it is of great interest to compare the prognostic value of the classical pathological prognosticators with that of proliferation markers, including phosphohistone H3 (PHH3), as new potential biomarkers for breast cancer.
PHH3 is a core histone protein seen during the late G2 and M phases, thus theoretically providing a stricter assessment for mitotic activity 17, and has been described as a potential surrogate marker for mitotic count in the recent literature. Recent studies have shown PHH3 IHC to be highly specific for phosphorylated histone H3, therefore serving as a good marker for mitoses18 Notably, PHH3 has been shown to be an useful adjunct for determining the “hot spot” for mitotic activity and therefore is recommended as a confirmatory staining technique for mitoses in the diagnosis of thin melanoma.19Additionally, the use of PHH3 IHC showed improved interobserver agreement in mitotic count and final grade assessment compared to H&E in well-differentiated neuroendocrine tumors of the pancreas20 More recently, degree of nuclear PHH3 expression was shown to be a strong prognostic marker for lymph node-negative breast cancer patients less than 55 years of age treated with systemic adjuvant chemotherapy and furthermore a stronger prognostic marker than ER/ PR status, Oncotype Dx, and Mammaprint21
PHH3 IHC stain analysis is known to have significantly increased interobserver agreement in mitotic rate calculation in recent studies6 In addition to the benefit of increasing the interobserver agreement for mitotic counts, PHH3 IHC also allows for more rapid objective determination of the mitotic count. For instance, with PHH3 IHC, apoptotic cell debris is more easily distinguished from mitoses and there is a greater chance for detecting mitotic cells with atypical morphology of metaphase or anaphase than by H&E stain alone.In contrast, prophase of mitotic figures is not easily detectable by H&E. However, analysis of PHH3 has been shown to increase the average mitotic rate by 86-200%22, 23, 24, 25, 26The IHC stain for PHH3 has been proposed as a potential surrogate marker for mitotic count, and it has previously been studied in various tumor types including melanoma19, 27, meningioma28, pulmonary carcinoid29, and well-differentiated neuroendocrine tumors of the pancreas30. In addition, PHH3 analysis is less time-consuming and easier to interpret than conventional mitotic counting by H&E stain.
In breast carcinoma, an early study on the use of PHH3 detected a strong correlation between PHH3‐stained and H&E‐stained mitotic counts in a series of 39 invasive breast carcinoma cases31This study found that the detection of mitotic figures with PHH3 IHC was more sensitive than H&E staining in all tumor grades, especially in grades 2 and 3. The increased sensitivity was attributed to the fact that prophase figures are more readily detected in PHH3‐stained sections, whereas they are not taken into account by H&E staining. The study also found that PHH3 IHC had utility in detecting mitotic cells in high‐grade tumors even in areas with dense cellularity and numerous apoptotic bodies or necrotic cells. Similar to our findings, a higher number of mitotic figures were detected by PHH3 IHC compared to conventional H&E. However, compared to our study, only three cases in the early study showed an upgrade in the mitotic score, including an upgrade from grade 1 to grade 2 in one case, and from grade 2 to grade 3 in two cases. 31A more recent study on 97 consecutive cases of invasive breast carcinoma demonstrated a significant proportion of cases with upgraded mitotic scores utilizing PHH3 versus H&E, similar to our study32
PHH3 IHC has also shown a stronger correlation to mitotic count than Ki-67. 34In this study, 49 consecutive cases of invasive breast carcinoma with H&E, PHH3, and Ki-67-stained slides were examined utilizing an automated digital image analysis over one defined tumor area. The study found the strongest correlation between H&E mitotic count and PHH3 counts, while correlations with Ki-67 were weaker versus PHH3 and H&E mitotic count. The authors suggest that this weaker correlation is attributable to the fact that Ki-67 measures multiple phases of the cell cycle, and cell cycle time can vary significantly among various tumor types. In our study, 22% of cases with Ki-67 IHC showed no concordance with either one of H&E or PHH3 mitotic scores, further suggesting that Ki-67 has a weaker correlation to either H&E or PHH3.
Schwartz et al. studied the largest number of breast cancer cases from the SEER program and concluded that the histologic grade is the most important prognostic factor for overall survival despite the tumor size and nodal status, thereby highlighting the significance of an accurate histologic grade.33 Other studies also demonstrated the importance of PHH3 stain in breast cancers between different age groups34, 35
Our study confirms that PHH3 is a more sensitive marker for mitotic figures than H&E by detecting an overall increase in the number of mitotic figures. Furthermore, PHH3 changed the mBR grade in 24% of our total cases and demonstrated a stronger correlation with the Ki-67 index. Although the majority of cases showed no change in mBR grade when using PHH3 mitotic scores, 23% of the total cases had mBR upgrade with PHH3 as compared to the 1% of total cases that showed mBR downgrade, suggesting that mitotic count by H&E alone may undergrade some tumors. In our study, the majority of cases with upgrading were a result of movement from grade 1 to grade 2 (52% of the upgraded cases) or grade 2 to grade 3 (41% of the upgraded cases), suggesting that PHH3 staining can facilitate the determination of the final mitotic score in borderline cases. For this reason, we recommend the use of PHH3 as an additional biomarker in the evaluation of invasive breast carcinomas that are grade 1 and 2 by identifying mitotic “hot spots.”
When comparing H&E and PHH3 staining with Ki-67, we found both H&E and PHH3 mitotic scores to correlate with Ki-67 in 51% of cases. Ki-67 had better correlation with PHH3 (19% of cases) over H&E (8% of cases). Twenty-two percent of cases showed no concordance between H&E, PHH3, or Ki-67. Reasons for this discordance may be multifactorial, including positive staining for Ki-67 by intratumoral lymphocytes, difficulties in identifying mitotic figures by H&E, and apoptotic bodies mimicking mitotic figures by H&E. Additionally, Ki-67 as an overall proliferation marker is non-specific and accounts for all phases of the cell cycle apart from G0, whereas mitotic figures by H&E and PHH3 positive nuclei account for cells only in the M phase.
Similar to previous studies, we find PHH3 to allow for a more sensitive and practical approach in the evaluation for interphase and prophase mitotic figures, which are difficult to identify on H&E-stained slides. PHH3 IHC is particularly useful as the evaluation of mitotic figures can be impaired by multiple variables, including fixation artifacts and confounding tumor properties such as necrosis, apoptosis, inflammation, and fibrosis, all of which may result in low interobserver reproducibility. While our study is not the first to demonstrate the utilization of PHH3 IHC staining in breast cancer, we further substantiate the growing amount of literature on how PHH3 can be routinely used for the accurate determination of mitotic counts which influences histologic grade. Further studies on the prognostic value of PHH3 in comparison to Ki-67 on a larger scale may be of value in the future.
The significance of using PHH3 IHC in our study is that 48 out of 451 cases (10.6%) of cases were upgraded either from grade 2 to grade 3 or grade 1 to grade 3. Patients with grade 3 tumors, almost invariably are offered chemotherapy, whereas patients with lower grade tumors will often have additional testing (such as Oncotype DX) to help make the decision whether to proceed with chemotherapy. In our study, approximately 10.6% of patients had a significant upgrade that may change the patient's treatment plans.
A limitation of our study is that although PHH3 in breast carcinoma allows for a more sensitive and practical approach in the identification of mitotic figures and PHH3 IHC is useful as a confirmatory tool in assessing the final mitotic score for more accurate breast carcinoma scores, our data lacks clinical outcome. Our data was taken from 2013-2014 patient samples when PHH3 IHC stain was made available in our laboratory and hence a long term follow-up is needed to verify the clinical outcome with PHH3 data. Clinical follow-up studies with PHH3 data will be of particular relevance to further validate the use of PHH3 IHC as a universal breast biomarker in the evaluation of invasive breast carcinoma. Consideration for revisiting breast carcinoma grading criteria utilizing the PHH3 stain may be necessary after evaluation of PHH3's utility in clinical practice, including a possible prognostic role.
Conclusion
In conclusion, the use of PHH3 IHC in breast carcinoma allows for a more practical and sensitive approach in the identification of mitotic figures. By increasing the mBR grade in a relatively large percentage of cases (23% of total cases in our study), PHH3 (versus H&E mitotic count) may be useful as a confirmatory tool in assessing the final mitotic score for more accurate determination of the mBR grade. Additionally, PHH3 IHC allows for easier and more rapid detection of mitotic figures and in identifying mitotic “hot spots,” which would be the ideal areas to perform the mitotic counts. Further studies on interobserver reproducibility in mitotic rate assessment and grade assignment, as well as patient outcomes, may further validate the use of PHH3 as a universal biomarker in the assessment of breast carcinoma.
Acknowledgements
We thank the UCLA Department of Pathology and Laboratory Medicine for their generous support. No funding was received for this study.
Financial Support:
No financial support was received for this study.
Disclaimers:
The authors have nothing to disclose.
Ethics
This study was approved by the UCLA Institutional Review Board.
Abbreviations
PHH3: Phosphohistone H3
IHC: immunohistochemical
References
- 1.V Le Doussal, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F. (1989) Prognostic value of histologic grade nuclear components of Scarff-Bloom-Richardson (SBR). An improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. , Cancer 64, 1914-1921.
- 2.B W Davis, R D Gelber, Goldhirsch A, W H Hartmann, G W Locher. (1986) Prognostic significance of tumor grade in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. , Cancer 58, 2662-2770.
- 3.C W Elston, I O Ellis. (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. , Histopathology 19, 403-410.
- 4.H J Bloom, W. (1957) Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. , Br. J. Cancer 11, 359-377.
- 5.Boiesen P, P O Bendahl, Anagnostaki L, Domanski H, Holm E. (2000) Histologic Grading in Breast Cancer: Reproducibility Between Seven Pathologic Departments. Acta Oncol. , (Madr) 39, 41-45.
- 6.Schimming T T, Grabellus F, Roner M, Pechlivanis S, Sucker A. (2012) pHH3 Immunostaining improves interobserver agreement of mitotic index in thin melanomas. , Am J Dermatopathol 34, 266-269.
- 7.Paik S, Shak S, Tang G, Kim C, Baker J. (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. , N. Engl. J. Med 351, 2817-2826.
- 8.Andre F, Khalil A, Slimane K, Massard C, Mathieu M C. (2005) Mitotic index and benefit of adjuvant anthracycline-based chemotherapy in patients with early breast cancer. , Journal of Clinical Oncology 23, 2996-3000.
- 9.Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J. (1991) Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. , Cytometry 12, 42-49.
- 10.Git A, Spiteri I, Blenkiron C, M J Dunning, J C Pole. (2008) PMC42, a breast progenitor cancer cell line, has normal-like mRNA and microRNA transcriptomes. , Breast Cancer Res 10, 54.
- 11.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D. (2008) Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. , Clin Cancer Res 14, 5158-5165.
- 12.Lo S S, Mumby P B, Norton J, Rychlik K, Smerage J. (2010) Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. , J Clin Oncol 28, 1671-1676.
- 13.Vijver M J van de, Y D He, J van’t Veer L, Dai H, A. (2002) A gene expression signature as a predictor of survival in breast cancer. , N Engl J Med 347, 1999-2009.
- 14.J P Baak, Gudlaugsson E, Skaland I, L H Guo, Klos J. (2009) Proliferation is the strongest prognosticator in node-negative breast cancer: significance, error sources, alternatives and comparison with molecular prognostic markers. , Breast Cancer Res Treat 115, 241-254.
- 15.Klintman M, Strand C, Ahlin C, Beglerbegovic S, M L Fjällskog et al.(December4,2013)The prognostic value of mitotic activity index (MAI). Phosphohistone H3 (PPH3), cyclin B1, cyclin A, and Ki67, alone and in combinations, in node-negative premenopausal breast cancer.PLoS One.0.1371/journal.pone.0081902 .
- 16.R I Nicholson, Bouzubar N, K J Walker, McClelland R, A R Dixon. (1991) Hormone sensitivity in breast cancer: influence of heterogeneity of oestrogen receptor expression and cell proliferation. , Eur J Cancer 27, 908-913.
- 17.Shibata K, Inagaki M, Ajiro K. (1990) Mitosis-specific histone H3 phosphorylation in vitro in nucleosome structures. , Eur. J. Biochem.192 87-93.
- 18.Hendzel M J, Wei Y, Mancini M A. (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. , Chromosoma 106, 348-360.
- 19.Thareja S, J S Zager, Sadhwani D, Thareja S, Chen R. (2014) Analysis of tumor mitotic rate in thin metastatic melanomas compared with thin melanomas without metastasis using both the hematoxylin and eosin and anti-phosphohistone 3 IHC stain. , Am. J. Dermatopathol 36, 64-67.
- 20.S M Voss, M P Riley, P M Lokhandwala, Wang M, Yang Z. (2015) Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. , Am. J. Surg. Pathol 39, 13-24.
- 21.Jonsdottir K, Assmus J, Slewa A, Gudlaugsson E, Skaland I. (2014) Prognostic value of gene signatures and proliferation in lymph-node-negative breast cancer.PLoS One.10.1371/journal.pone.0090642.
- 22.Angi M, Damato B, Kalirai H, Dodson A, Taktak A. (2011) assessment of mitotic count in uveal melanoma.Doi: 10.1111/j.1755-3768.2009.01769.x.Acta Ophthalmol.
- 23.D J Casper, K I Ross, J L Messina, V K Sondak, C N Bodden. (2010) Use of anti-phosphohistone H3 immunohistochemistry to determine mitotic rate in thin melanoma. , Am J Dermatopathol 32, 650-654.
- 24.Ladstein R G, Bachmann I M, Straume O, Akslen L A.(April14,2010) Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. , BMC Cancer.Doi: 10-1186.
- 25.Glatz K, Hartmann C, Antic M, Kutzner H. (2010) Frequent mitotic activity in banal melanocytic nevi uncovered by immunohistochemical analysis. , Am J Dermatopathol 32, 643-649.
- 26.Nasr M R, El-Zammar O. (2008) Comparison of pHH3, Ki-67, and survivin immunoreactivity in benign and malignant melanocytic lesions. , Am J Dermatopathol 30, 117-122.
- 27.P S Nielsen, Riber-Hansen R, T O Jensen, Schmidt H, Steiniche T. (2013) Proliferation indices of phosphohistone H3 and Ki67: strong prognostic markers in a consecutive cohort with stage I/II melanoma. , Mod. Pathol 26, 404-413.
- 28.Ribalta T, I E McCutcheon, K D Aldape, J M Bruner, G N Fuller. (2004) The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO. , Am. J. Surg. Pathol 28, 1532-6.
- 29.Tsuta K, Liu D C, Kalhor N, Wistuba Moran CA. (2011) Using the mitosis-specific marker anti-phosphohistone H3 to assess mitosis in pulmonary neuroendocrine carcinomas. , Am. J. Clin. Pathol 136, 252-259.
- 30.S M Voss, M P Riley, P M Lokhandwala, Wang M, Yang Z. (2015) Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. , Am. J. Surg. Pathol 39, 13-24.
- 31.Bossard C, Jarry A, Colombeix C, Bach-Ngohou K, Moreau A. (2006) Phosphohistone H3 labeling for histoprognostic grading of breast adenocarcinomas and computer-assisted determination of mitotic index. , J. Clin. Pathol 59, 706-10.
- 32.L H, Yang H, Bigras G. (2014) Current breast cancer proliferative markers correlate variably based on decoupled duration of cell cycle phases. , Sci. Rep 4, 5122.
- 33.A M Schwartz, D E Henson, Chen D, Rajamarthandan S. (2014) Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 cases of breast cancer from the SEER Program. Arch Pathol Lab Med. 138, 1048-52.
