Abstract
The chemical and structural similarities of calcium orthophosphates (abbreviated as CaPO4)to the mineral composition of natural bones and teeth have made them a good candidate for bone tissue engineering applications. Nowadays, a variety of natural or synthetic CaPO4-based biomaterials is produced and has been extensively used for dental and orthopedic applications. Despite their inherent brittleness, CaPO4 materials possess several appealing characteristics as scaffold materials. Namely, their biocompatibility and variable stoichiometry, thus surface charge density, functionality and dissolution properties, make them suitable for both drug and growth factor delivery. Therefore, CaPO4, especially hydroxyapatite (HA) and tricalcium phosphates (TCPs), have attracted a significant interest in simultaneous use as bone grafts and drug delivery vehicles. Namely, CaPO4-based three-dimensional (3D) scaffolds and/or carriers have been designed to induce bone formation and vascularization. These scaffolds are usually porous and harbor various types of drugs, biologically active molecules and/or cells. Over the past few decades, their application as bone grafts in combination with stem cells has gained much importance. This review discusses the source, manufacturing methods and advantages of using CaPO4 scaffolds for bone tissue engineering applications. Perspective future applications comprise drug delivery and tissue engineering purposes.
Author Contributions
Academic Editor: Benaka Prasad S B, Jain University, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Sergey V. Dorozhkin
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Bones are organs and the living support structures that give the body form and shape. In the musculoskeletal system, bones act as the levers and pivots that control for direction and range of movement. Bones also function to protect our vital organs and act as a reservoir for critical vitamins and nutrients such as calcium. Bone tissues have an innate ability to remodel and regenerate themselves; however, when defects appear to be too large or when the normal repair process has been interrupted or disregulated, bones become unable to completely heal without external intervention 1.
In general, utilization of fixation devices and implants, such as fixation plates, intramedullary nails etc., often in combination with autografts/allografts and artificial bone substitutes appears to be the standard intervention strategy for complicated fractures. The benefits of using autografts are obvious. Briefly, they provide a matrix to support cell attachment and migration to generate new bone (osteoconductivity), contain growth factors and proteins that stimulate osteogenic differentiation (osteoinductivity), as well as contain live cells that act as a source for new bone formation (osteogenesis). A constraint of autografts is the limited availability of tissue, the frequent requirement of a second surgical site (e.g., iliac crest) and the subsequent risk of donor site morbidity. An alternative to autografts are allografts, which can be derived from donor patients or other species (that is xenografts, such as bovine bone chips). Allografts are more readily available and range from small bone chips to whole bone segments. As a result, allografts are osteoconductive, can be osteoinductive (if growths factors are preserved during the treatment process), but are not osteogenic due to lack of living cells. The complication rate and requirement for surgical revision have been reported to be significantly higher in bone allografts compared to autografts due to poor remodeling capability. In addition, there is a risk of disease transmission and immune reaction, associated with allografts 2.
Therefore, clinicians are looking to emerging fields, such as tissue engineering and clinical regenerative medicine, to overcome the limitations with current intervention strategies associated with complicated bone defects. Tissue engineering involves the use of scaffolds, biochemical factors or cells to restore the structure and function of tissues that have been damaged by disease or trauma. Within this field, bone tissue engineering is one of the most developed and deployed research areas 3.
The purpose of this review is to evaluate the role and impact of one particular subset of biomaterials in tissue engineering applications, namely: calcium orthophosphate (CaPO4) scaffolds for hard tissue regeneration. The focus is on the recent developments of new formulations and their formation into scaffolds with the requisite anatomical shape and architecture. Methods that can be used to manipulate the materials structure and the variables that affect the materials performance in these applications are analyzed.
General Knowledge and Definitions
According to the available literature, the term “tissue engineering” first appeared in 1984 in a paper by Wolter and Meyer 4 to overcome the major limitations of tissue grafting. Further, it was officially coined in 1988 at a meeting of the U.S. National Science Foundation as “the application of the principles and methods of engineering and life sciences towards the fundamental understanding of structure/function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain or improve functions” 5. Thus, this field of science started more than two decades ago 6, 7 and the famous publication by Langer and Vacanti 8 has greatly contributed to the promotion of tissue engineering research worldwide. Since then, the better definitions for tissue engineering have been sought and the following ones were proposed by Prof. David Williams (that time he was the Editor-in-Chief of Biomaterials journal) in 1999 and 2008, respectively: “The persuasion of the body to heal itself, through the delivery to the appropriate sites of molecular signals, cells and/or supporting structures” (1999) and “Tissue engineering is the creation of new tissue for the therapeutic reconstruction of the human body, by the deliberate and controlled stimulation of selected target cells through a systematic combination of molecular and mechanical signals” (2008) 9.
Tissue engineering may be achieved through several different routes but there is a basic paradigm of ex vivo tissue regeneration, which may serve as a template, in which there is a progression from cell sourcing through cell manipulation and signaling to tissue expression and construct formation, followed by implantation into the host and its full incorporation into that host. In the centre of this paradigm is the seeding of the required cells into a biomaterial scaffold or matrix, wherein they produce the new tissue. Usually, although not necessarily, the biomaterial is required to degrade or dissolve as the new tissue forms 9.
Nevertheless, tissue/organ repair has been the ultimate goal of surgery from ancient times to nowadays 10, 11. The repair has traditionally taken two major forms: tissue grafting followed by organ transplantation and alloplastic or synthetic material replacement. Both approaches, however, have limitations. Grafting requires second surgical sites with associated morbidity and is restricted by limited amounts of material, especially for organ replacement. Synthetic materials often integrate poorly with host tissue and fail over time due to wear and fatigue or adverse body response 12. In addition, all modern orthopedic implants lack three of the most critical abilities of living tissues: (i) self-repairing; (ii) maintaining of blood supply; (iii) self-modifying their structure and properties in response to external aspects such as a mechanical load 13. Needless to mention, that bones not only possess all of these properties but, in addition, they are self-generating, hierarchical, multifunctional, nonlinear, composite and biodegradable; therefore, the ideal artificial bone grafts must possess similar properties 14.
Bone substitute materials can be defined as “a synthetic, inorganic or biologically organic combination – biomaterial – which can be inserted for the treatment of a bone defect instead of autogenous or allogenous bone” 15. This is a broad definition and a variety of materials have been used over time to substitute or generate bone tissue. In order to be ultimately applicable in the human body, one of the key requirements is that the bone substitute materials must be non-carcinogenic, non-toxic, non-teratogenic and possess a high cell and tissue biocompatibility (ability of a material to perform with an adequate response in a specific application). Since the inorganic part of bones and teeth of mammals consist of CaPO4 of biological origin, synthetically manufactured CaPO4 (a list of the known CaPO4, including their standard abbreviations and major properties, is summarized in Table 116, 17), appear to fulfill all these requirements.
Table 1. Existing calcium orthophosphates and their major properties 1617.| Ca/P molar ratio | Compounds and their typical abbreviations | Chemical formula | Solubility at 25 ºC, -log(K s ) | Solubility at 25 ºC, g/L | pH stability range in aqueous solutions at 25°C |
| 0.5 | Monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 1.14 | ~ 18 | 0.0 – 2.0 |
| 0.5 | Monocalcium phosphate anhydrous (MCPA or MCP) | Ca(H2PO4)2 | 1.14 | ~ 17 | c |
| 1.0 | Dicalcium phosphate dihydrate (DCPD), mineral brushite | CaHPO4·2H2O | 6.59 | ~ 0.088 | 2.0 – 6.0 |
| 1.0 | Dicalcium phosphate anhydrous (DCPA or DCP), mineral monetite | CaHPO4 | 6.90 | ~ 0.048 | c |
| 1.33 | Octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4·5H2O | 96.6 | ~ 0.0081 | 5.5 – 7.0 |
| 1.5 | α-Tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 25.5 | ~ 0.0025 | a |
| 1.5 | β-Tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | 28.9 | ~ 0.0005 | a |
| 1.2 – 2.2 | Amorphous calcium phosphates (ACP) | CaxHy(PO4)z·nH2O, n = 3 – 4.5; 15 – 20 % H2O | b | b | ~ 5 – 12d |
| 1.5 – 1.67 | Calcium-deficient hydroxyapatite (CDHA or Ca-def HA)[e] | Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0<x<1) | ~ 85 | ~ 0.0094 | 6.5 – 9.5 |
| 1.67 | Hydroxyapatite (HA, HAp or OHAp) | Ca10(PO4)6(OH)2 | 116.8 | ~ 0.0003 | 9.5 – 12 |
| 1.67 | Fluorapatite (FA or FAp) | Ca10(PO4)6F2 | 120.0 | ~ 0.0002 | 7 – 12 |
| 1.67 | Oxyapatite (OA, OAp or OXA)[f], mineral voelckerite | Ca10(PO4)6O | ~ 69 | ~ 0.087 | a |
| 2.0 | Tetracalcium phosphate (TTCP or TetCP), mineral hilgenstockite | Ca4(PO4)2O | 38 – 44 | ~ 0.0007 | a |
In 2007, a “diamond concept” of bone tissue engineering was proposed as a “standard tissue engineering approach to provide solutions for impaired fracture healing, bone restoration and regeneration” 18, 19, which has become widely accepted and acknowledged in the field of bone tissue engineering. According to this concept, the ideal bone tissue engineering approaches should utilize an osteoconductive (i.e., guiding bone ingrowth) three-dimensional (3D) structure (e.g., scaffold, matrices), contain osteogenic (i.e., bone forming) cells and osteoinductive (i.e., inducing bone formation) factors, but must also have sufficient mechanical properties and promote vascularization. Since cells and osteoinductive factors do not contain CaPO4, let me discuss scaffolds only.
According to Wikipedia, the free encyclopedia, a term “scaffold” has several definitions depending on the specific application. For example, in construction, it is “a temporary structure that supports workers and equipment above the ground or floor”. In chemistry, it is “a structure that is used to hold up or support another material, such as a drug, crystal or protein”. In tissue engineering, it is “an artificial structure capable of supporting three-dimensional tissue formation” 20. In spite of the differences, all these definitions contain the meaning on “a structure that supports”, which is the key. Since bone substitute materials are always implanted, the bone grafting scaffolds must be manufactured from the materials, which are well tolerated by the human bodies, among which CaPO4 appear to be most promising candidates.
Scaffolds and their Major Properties
Since the shortage of donor tissues or organs appears to be the biggest issue for organ transplantation, it would be very convenient to both patients and physicians if devastated tissues or organs of patients can be regenerated by simple cell injections to the target sites. Unfortunately, such cases are rare. The majority of large-sized tissues and organs with distinct 3D form require a support for their formation from cells. The support is called scaffold, template and/or artificial extracellular matrix 21, 22, 23, 24, 25, 26, 27, 28. The major function of scaffolds is to balance temporary mechanical functions with mass transport to aid biological delivery and tissue regeneration 12. Thus, scaffolds play a role of temporary extracellular matrixes and assist proliferation, differentiation and biosynthesis of cells on the surface of their own. In addition, scaffolds placed at the regeneration sites prevent disturbing cells from invasion into the sites of action 29, 30. However, for the future of tissue engineering, the term ‘template’ might become more suitable because, according to David F. Williams, the term scaffold “conveys an old fashioned meaning of an inert external structure that is temporarily used to assist in the construction or repair of inanimate objects such as buildings, taking no part in the characteristics of the finished product.” 31 p. 1129.
Therefore, the idea behind tissue engineering is to create or engineer autografts by either expanding autologous cells in vitro guided by a scaffold or implanting an acellular template in vivo and allowing the patient’s cells to repair the tissue guided by the scaffold. The first phase is the in vitro formation of a tissue construct by placing the chosen cells and scaffolds in a metabolically and mechanically supportive environment with growth media (in a bioreactor), in which the cells proliferate and elaborate extracellular matrix. It is expected that cells infiltrate into the porous matrix and consequently proliferate and differentiate therein 32, 33. In the second phase, the construct is implanted in the appropriate anatomic location, where remodeling in vivo is intended to recapitulate the normal functional architecture of an organ or a tissue 34, 35. The key processes occurring during both in vitro and in vivo phases of the tissue formation and maturation are: (1) cell proliferation, sorting and differentiation, (2) extracellular matrix production and organization, (3) biodegradation of the scaffold, (4) remodeling and potentially growth of the tissue 36.
To achieve the goal of tissue reconstruction, the scaffolds (templates) must meet a number of the specific requirements 21, 27, 31. First, for an appropriate use in the human body, all scaffolds need to be made from highly biocompatible materials that do not elicit any adverse permanent immune responses in the host tissue after local implantation. The potential group of biomaterials comprise bioceramics, biodegradable polymers and their biocomposites 37. Further, a reasonable surface roughness is necessary to facilitate cell seeding and fixation 38, 39, 40, 41, 42, 43. In addition, artificial scaffolds must bond to the host tissues without formation of any type of scar tissues, creating a stable interface. A high porosity and an adequate pore dimensions (Table 2) are very important to allow cell migration, vascularization, as well as a diffusion of nutrients 44, 45. A sufficient mechanical strength and stiffness are mandatory to oppose contraction forces and later for the remodeling of damaged tissues 46, 47. A French architect Robert le Ricolais (1894 – 1977) stated: “The art of structure is where to put the holes”. Therefore, to enable proper tissue ingrowth, vascularization and nutrient delivery, scaffolds should have a highly interconnected porous network, formed by a combination of macro- and micropores, in which more than ~ 60 % of the pores should have a size ranging from ~ 150 μm to ~ 400 μm and at least ~ 20 % should be smaller than ~ 20 μm 44, 45, 48, 49, 50, 51, 52, 53, 54, 55, 56. What’s more, the entire geometry of porous scaffolds appears to significantly influence the cellular response and the rate of bone tissue regeneration. Namely, rates of tissue generation were found to increase with curvature and appeared to be much larger on concave surfaces as compared to convex and planar ones 57. In addition, scaffolds must be manufactured from the materials with controlled biodegradability and/or bioresorbability, such as CaPO4, so that a new bone will eventually replace the scaffold 23, 50, 58. Furthermore, the degradation by-products of scaffolds must be non-cytotoxic. More to the point, the resorption rate has to coincide as much as possible with the rate of bone formation (i.e., between a few months and about 2 years) 59. This means that while cells are fabricating their own natural matrix structure around themselves, the scaffold is able to provide a structural integrity within the body and eventually it will break down leaving the newly formed tissue that will take over the mechanical load. However, one should bear in mind that the scaffold’s architecture changes with the degradation process and the degradation by-products affect the biological response. Besides, scaffolds should be easily fabricated into a variety of shapes and sizes 60, be malleable to fit irregularly shaped defects, while the fabrication processes should be effortlessly scalable for mass production. In many cases, ease of processability, as well as easiness of conformation and injectability, such as self-setting CaPO4 formulations possess 61, can determine the choice of a certain biomaterial. Finally, sterilization with no loss of properties is a crucial step in scaffold production at both a laboratory and an industrial level 23, 24, 25. Thus, each scaffold (template) should fulfill many functions before, during and after implantation.
Table 2. A hierarchical pore size distribution that an ideal scaffold should exhibit 45.| Pore sizes of a 3D scaffold | A biochemical effect or function |
|---|---|
| < 1 μm | Interaction with proteins |
| Responsible for bioactivity | |
| 1 – 20 μm | Type of cells attracted |
| Cellular development | |
| Orientation and directionality of cellular ingrowth | |
| 100 – 1000 μm | Cellular growth |
| Bone ingrowth | |
| Predominant function in the mechanical strength | |
| > 1000 μm | Implant functionality |
| Implant shape | |
| Implant esthetics |
In order to achieve the desired properties at the minimum expenses, the production process should be optimized 62. The main goal is to develop a high potential synthetic bone substitute (so called “smart scaffold”) which will not only promote osteoconduction but also osteopromotion, i.e. the ability to enhance of osteoinduction 63. In the case of CaPO4, a smart scaffold represents a biphasic (HA/β-TCP ratio of 20/80) formulation with a total porosity of ~ 73 %, constituted of macropores (> 100 µm), mesopores (10 – 100 µm) and a high content (~ 40 %) of micropores (< 10 µm) with the crystal dimensions within < 0.5 to 1 µm and the specific surface area ~ 6m2/g 64. With the advent of CaPO4 in tissue engineering, the search is on for the ultimate option consisting of a synthetic smart scaffold impregnated with cells and growth factors. Figure 1 schematically depicts a possible fabrication process of such item that, afterwards, will be implanted into a living organism to induce bone regeneration 65.
Figure 1.A schematic view of a third generation biomaterial, in which porous CaPO4 bioceramics acts as a scaffold or a template for cells, growth factors, etc. Reprinted from Ref. 65 with permission.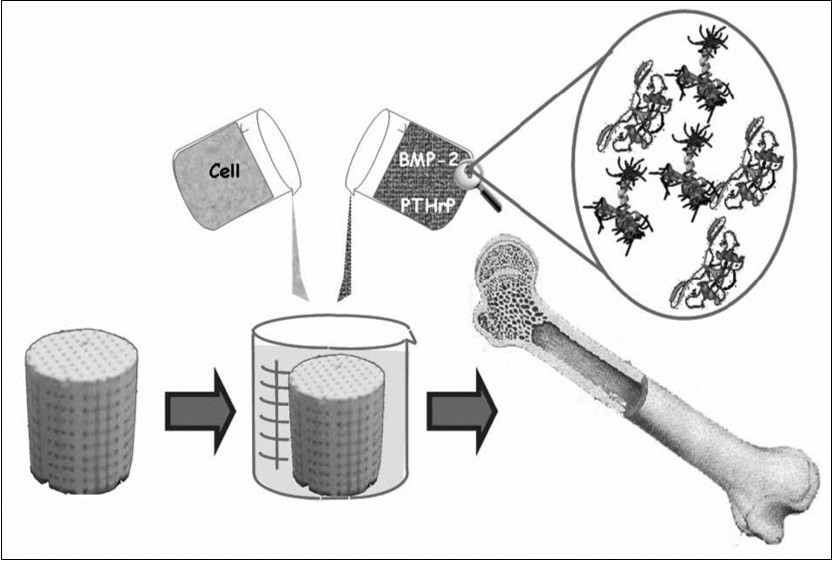
To finalize this topic, one should mention on fundamental unfeasibility to create so-called “ideal scaffold” for bone grafting. Since bones of human skeleton have very different dimensions, shapes and structures depending on their functions and locations, synthetic bone grafts of various sizes, shapes, porosity, mechanical strength, composition and resorbability appear to be necessary. Therefore, bioceramic scaffolds of 0 to 15 % porosity are used as both ilium and intervertebral spacers, where a high strength is required, those of 30 to 40 % porosity are useful as spinous process spacer for laminoplasty, where both bone formation and middle strength are necessary, while ones of 40 to 60 % porosity appear to be useful for the calvarias plate, where a fast bone formation is needed (Figure 2) 66. Furthermore, defining the optimum parameters for artificial scaffolds is in fact an attempt to find a reasonable compromise between various conflicting functional requirements. Namely, an increased mechanical strength of bone substitutes requires solid and dense structures, while colonization of their surfaces by cells requires interconnected porosity. Additional details and arguments on this subject are well described elsewhere 67, in which the authors concluded: “there is enough evidence to postulate that ideal scaffold architecture does not exist.” (p. 478).
Figure 2.A schematic drawing presenting the potential usage of bioceramic scaffolds with various degrees of porosity. Reprinted from Ref. 66 with permission.
CaPO4 Bioceramics
Currently, CaPO4 bioceramics can be prepared from various sources 68, 69, 70, 71, 72, 73, 74. Nevertheless, up to now, all attempts to synthesize bone replacement materials for clinical applications featuring the physiological tolerance, biocompatibility and a long-term stability have had only a relative success; this clearly demonstrates both the superiority and a complexity of the natural structures 14.
In general, a characterization of CaPO4 bioceramics should be performed from various viewpoints such as the chemical composition (including stoichiometry and purity), homogeneity, phase distribution, morphology, grain sizes and shape, grain boundaries, crystallite size, crystallinity, pores, cracks, surface roughness, etc. Among the known types of CaPO4 (Table 1), the vast majority of CaPO4 bioceramics is based on HA 75, 76, 77, 78, 79, 80, both types of TCP 75, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 and various multiphasic formulations thereof 92. Biphasic formulations (commonly abbreviated as BCP – biphasic calcium phosphate) are the simplest among the latter ones. They include β-TCP + HA 93, 94, 95, 96, 97, 98, 99, 100, 101, α-TCP + HA 102, 103, 104 and biphasic TCP (commonly abbreviated as BTCP) consisting of α-TCP and β-TCP 105, 106, 107, 108, 109, 110. In addition, triphasic formulations (HA + α-TCP + β-TCP) have been prepared as well 111, 112, 113, 114. Further details on this topic might be found in a special review 92. Leaving aside a big subject of DCPD-forming self-setting formulations 61, 115, one should note that just a few publications on bioceramics, prepared from other types of CaPO4, are available 116, 117, 118, 119, 120, 121, 122, 123, 124.
The preparation techniques of various CaPO4 have been extensively reviewed in literature 125, 126, 127, 128, 129 where the interested readers are referred to. Briefly, when compared to both α- and β-TCP, HA is a more stable phase under the physiological conditions, as it has a lower solubility (Table 1) and, thus, slower resorption kinetics 130, 131, 132. Therefore, the BCP concept is determined by the optimum balance of a more stable phase of HA and a more soluble TCP. Due to a higher biodegradability of the α- or β-TCP component, the reactivity of BCP increases with the TCP/HA ratio increasing. Thus, in vivo bioresorbability of BCP can be controlled through the phase composition 94. Similar conclusions are also valid for the biphasic TCP (in which α-TCP is a more soluble phase), as well as for both triphasic (HA, α-TCP and β-TCP) and yet more complex formulations 92.
As implants made of sintered HA are found in bone defects for many years after implantation (Figure 3, bottom), bioceramics made of more soluble types of CaPO475,81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 133,134 are preferable for the biomedical purposes (Figure 3, top). Furthermore, the experimental results showed that BCP had a higher ability to adsorb fibrinogen, insulin or type I collagen than HA 135. Thus, according to both observed and measured bone formation parameters, CaPO4 bioceramics have been ranked as follows: low sintering temperature BCP (rough and smooth) ≈ medium sintering temperature BCP ≈ TCP > calcined low sintering temperature HA > non-calcined low sintering temperature HA > high sintering temperature BCP (rough and smooth) > high sintering temperature HA 136. This sequence has been developed in year 2000 and, thus, neither multiphase formulations, nor other CaPO4 have been included.
Figure 3.Soft X-ray photographs of the operated portion of the rabbit femur. Four weeks (a), 12 weeks (b), 24 weeks (c) and 72 weeks (d) after implantation of CDHA; 4 weeks (e), 12 weeks (f), 24 weeks (g) and 72 weeks (h) after implantation of sintered HA. Reprinted from Ref. 133 with permission.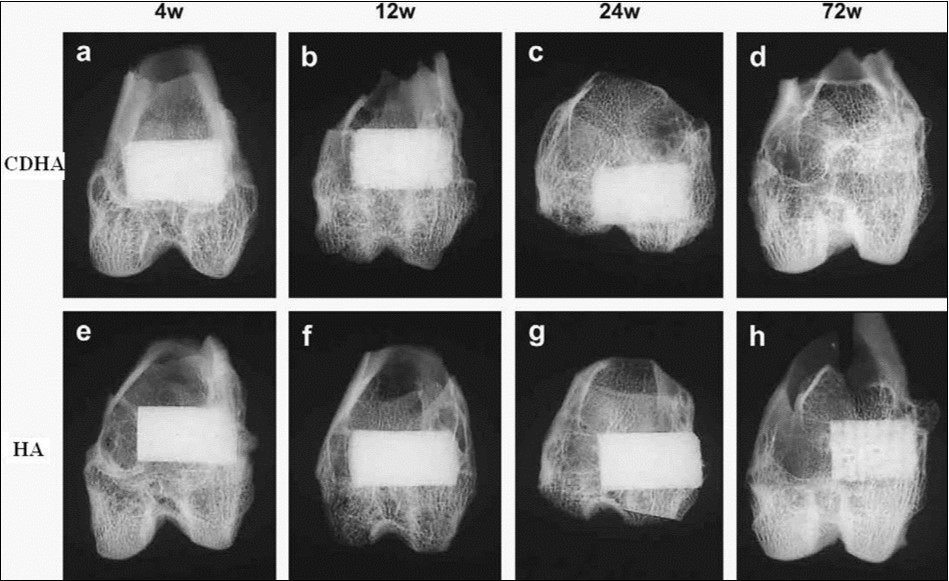
Scaffolds from CaPO4
Forming and Shaping
In order to fabricate scaffolds in progressively complex shapes, scientists are investigating the use of both old and new manufacturing techniques. These techniques range from an adaptation of the age-old pottery techniques to the newest manufacturing methods for high-temperature ceramic parts for airplane engines. Namely, reverse engineering 137, 138 and rapid prototyping 139, 140, 141 technologies have revolutionized a generation of physical models, allowing the engineers to efficiently and accurately produce physical models and customized implants with high levels of geometric intricacy. Combined with the computer-aided design and manufacturing (CAD/CAM), complex physical objects of the anatomical structure can be fabricated in a variety of shapes and sizes. In a typical application, an image of a bone defect in a patient can be taken and used to develop a 3D CAD computer model 142, 143, 144, 145, 146. Then a computer can reduce the model to slices or layers. Afterwards, 3D objects and coatings are constructed layer-by-layer using rapid prototyping techniques. The examples comprise fused deposition modeling 147, 148, selective laser sintering 149, 150, 151, 152, 153, 154, 155, laser cladding 156, 157, 158, 159, 3D printing and/or plotting 86, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, solid freeform fabrication 175, 176, 177, 178, 179, 180, 181, 182, 183 and stereolithography 184, 185, 186, 187. 3D printing and/or plotting of the CaPO4 -based self-setting formulations could be performed as well 172, 173. In the specific case of ceramic scaffolds, a sintering step is usually applied after printing the green bodies. Furthermore, a thermal printing process of melted CaPO4 has been proposed 188, while, in some cases, laser processing might be applied as well 189, 190. A schematic of 3D printing technique, as well as some 3D printed items are shown in Figure 410. A custom-made implant of actual dimensions would reduce the time it takes to perform the medical implantation procedure and subsequently lower the risk to the patient. Another advantage of a pre-fabricated, exact-fitting implant is that it can be used more effectively and applied directly to the damaged site rather than a replacement, which is formulated during surgery from a paste or granular material 176, 190, 191, 192.
Figure 4.A schematic of 3D printing and some 3D printed parts (fabricated at Washington State University) showing the versatility of 3D printing technology for ceramic scaffolds fabrication with complex architectural features. Reprinted from Ref. 10 with permission.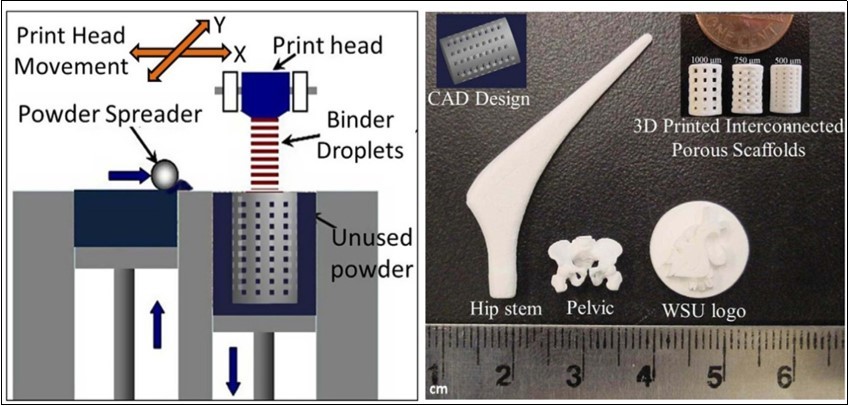
In addition to the aforementioned modern techniques, classical forming and shaping approaches are still widely used. The selection of the desired technique depends greatly on the ultimate application of scaffolds, e.g., whether they are for a hard-tissue replacement or an integration of the device within the surrounding tissues. In general, three types of the processing technologies might be used: (1) employment of a lubricant and a liquid binder with ceramic powders for shaping and subsequent firing; (2) application of self-setting and self-hardening properties of water-wet molded powders; (3) materials are melted to form a liquid and are shaped during cooling and solidification 193, 194, 195, 196. Since CaPO4 are either thermally unstable (MCPM, MCPA, DCPA, DCPD, OCP, ACP, CDHA) or have a melting point at temperatures exceeding ~ 1400 °C with a partial decomposition (α-TCP, β-TCP, HA, FA, TTCP), only the first and the second consolidation approaches are used to prepare bulk b ioceramics and scaffolds. The methods include uniaxial compaction 197, 198, 199, isostatic pressing (cold or hot) 100, 200, 201, 202, 203, 204, 205, 206, 207, granulation 208, 209, 210, 211, 212, 213, 214, loose packing 215, slip casting 88, 216, 217, 218, 219, 220, 221, gel casting 184, 222, 223, 224, 225, 226, 227, 228, 229, 230, pressure mold forming 231, 232, injection molding 233, 234, 235, polymer replication 236, 237, 238, 239, 240, 241, 242, 243, ultrasonic machining 244, extrusion 245, 246, 247, 248, 249, 250, 251, slurry dipping and spraying 252. Depending on the fabrication technique used, various parameters such as solid loading of the ceramic slurry, type and amount of additives (binders, surfactants, dispersants, etc.), temperature, etc. should be optimized to maximize the mechanical strength of the scaffolds. In addition, to form ceramic sheets from slurries, tape casting 225, 253, 254, 255, 256, 257, doctor blade 258 and colander methods can be employed 193, 194, 195, 196. Furthermore, flexible, ultrathin (of 1 to several microns thick), freestanding HA sheets were produced by a pulsed laser deposition technique, followed by thin film isolation technology 259. Various combinations of several techniques are also possible 90, 225, 260, 261, 262. More to the point, some of these processes might be performed under the electromagnetic field, which helps crystal aligning 217, 220, 263, 264, 265, 266. Finally, the prepared CaPO4 bioceramics might be subjected by additional treatments (e.g., chemical, thermal and/or hydrothermal ones) to convert one type of CaPO4 into another one 243.
To prepare bulk bioceramics, powders are usually pressed damp in metal dies or dry in lubricated dies at pressures high enough to form sufficiently strong structures to hold together until they are sintered 267. An organic binder, such as polyvinyl alcohol, helps to bind the powder particles altogether. Afterwards, the binder is removed by heating in air to oxidize the organic phases to carbon dioxide and water. Since many binders contain water, drying at ~ 100 °C is a critical step in preparing damp-formed pieces for firing. Too much or too little water in the compacts can lead to blowing apart the ware on heating or crumbling, respectively 193, 194, 195, 196. Furthermore, removal of water during drying often results in subsequent shrinkage of the product. In addition, due to local variations in water content, warping and even cracks may be developed during drying. Dry pressing and hydrostatic molding can minimize these problems 196. Finally, the manufactured green samples are sintered.
It is important to note that forming and shaping of any ceramic products require a proper selection of the raw materials in terms of particle sizes and size distribution. Namely, tough and strong scaffolds consist of pure, fine and homogeneous microstructures. To attain this, pure powders with small average size and high surface area must be used as the starting sources. However, for maximum packing and least shrinkage after firing, mixing of ~ 70 % coarse and ~ 30 % fine powders have been suggested 196. Mixing is usually carried out in a ball mill for uniformity of properties and reaction during subsequent firing. Mechanical die forming or sometimes extrusion through a die orifice can be used to produce a fixed cross-section.
Finally, to produce the accurate shaping, necessary for the fine design of scaffolds, machine finishing might be essential 144, 193, 268, 269. Unfortunately, cutting tools developed for metals are usually useless for bioceramics due to their fragility; therefore, grinding and polishing appear to be the convenient finishing techniques 144, 193. In addition, the surface of scaffolds might be modified by various supplementary treatments 270.
Sintering and Firing
A sintering (or firing) procedure appears to be of a great importance to manufacture bioceramic scaffolds with the required mechanical properties. Usually, this stage is carried out according to controlled temperature programs of electric furnaces in adjusted ambience of air with necessary additional gasses; however, always at temperatures below the melting points of the materials. The firing step can include temporary holds at intermediate temperatures to burn out organic binders 193, 194, 195, 196. The heating rate, sintering temperature and holding time depend on the starting materials. For example, in the case of HA, these values are in the ranges of 0.5 – 3 °C/min, 1000 – 1250 °C and 2 – 5 h, respectively 271. In the majority cases, sintering allows a structure to retain its shape. However, this process might be accompanied by a considerable degree of shrinkage 272, 273, 274, which must be accommodated in the fabrication process. For instance, in the case of FA sintering, a linear shrinkage was found to occur at ~ 715 °C and the material reached its final density at ~ 890 °C. Above this value, grain growth became important and induced an intra-granular porosity, which was responsible for density decrease. At ~ 1180 °C, a liquid phase was formed due to formation of a binary eutectic between FA and fluorite contained in the powder as impurity. This liquid phase further promoted the coarsening process and induced formation of large pores at high temperatures 275.
In general, sintering occurs only when the driving force is sufficiently high, while the latter relates to the decrease in surface and interfacial energies of the system by matter (molecules, atoms or ions) transport, which can proceed by solid, liquid or gaseous phase diffusion. Namely, when solids are heated to high temperatures, their constituents are driven to move to fill up pores and open channels between the grains of powders, as well as to compensate for the surface energy differences among their convex and concave surfaces (matter moves from convex to concave). At the initial stages, bottlenecks are formed and grow among the particles. Existing vacancies tend to flow away from the surfaces of sharply curved necks; this is an equivalent of a material flow towards the necks, which grow as the voids shrink. Small contact areas among the particles expand and, at the same time, a density of the compact increases and the total void volume decreases. As the pores and open channels are closed during a heat treatment, the particles become tightly bonded together and density, strength and fatigue resistance of the sintered object improve greatly. Grain-boundary diffusion was identified as the dominant mechanism for densification 276. Furthermore, strong chemical bonds are formed among the particles and loosely compacted green bodies are hardened to denser materials 193, 194, 195, 196. Further knowledge on the ceramic sintering process might be found elsewhere 277.
In the case of CaPO4, the earliest paper on their sintering was published in 1971 278. Since then, numerous papers on this subject were published and several specific processes were found to occur during CaPO4 sintering. Firstly, moisture, carbonates and all other volatile chemicals remaining from the synthesis stage, such as ammonia, nitrates and any organic compounds, are removed as gaseous products. Secondly, unless powders are sintered, the removal of these gases facilitates production of denser ceramics with subsequent shrinkage of the samples. Thirdly, all chemical changes are accompanied by a concurrent increase in crystal size and a decrease in the specific surface area. Fourthly, a chemical decomposition of all acidic orthophosphates and their transformation into other phosphates (e.g., 2HPO42- → P2O74- + H2O↑) takes place. Besides, sintering causes toughening 79, densification 80, 279, partial dehydroxylation (in the case of HA) 80, grain growth 276, 280, as well as it increases the mechanical strength 281, 282, 283. The latter events are due to presence of air and other gases filling gaps among the particles of un-sintered powders. At sintering, the gases move towards the outside of powders and green bodies shrink owing to decrease of distances among the particles. For example, sintering of a biologically formed apatites was investigated 284, 285 and the obtained products were characterized 286, 287. In all cases, the numerical value of Ca/P ratio in sintered apatites of biological origin was higher than that of the stoichiometric HA. One should mention that in the vast majority cases, CaPO4 with Ca/P ratio < 1.5 (Table 1) are not sintered, since these compounds are thermally unstable, while sintering of non-stoichiometric CaPO4 (CDHA and ACP) always leads to their transformation into various types of biphasic, triphasic and multiphase formulations 92.
An extensive study on the effects of sintering temperature and time on the properties of HA bioceramics revealed a correlation between these parameters and density, porosity, grain size, chemical composition and strength of the scaffolds 288. Namely, sintering below ~ 1000 °C was found to result in initial particle coalescence, with little or no densification and a significant loss of the surface area and porosity. The degree of densification appeared to depend on the sintering temperature whereas the degree of ionic diffusion was governed by the period of sintering 288. To enhance sinterability of CaPO4, a variety of sintering additives might be added 289, 290, 291, 292.
Solid-state pressureless sintering is the simplest procedure. For example, HA scaffolds can be pressurelessly sintered up to the theoretical density at 1000 – 1200 °C. Processing at even higher temperatures usually lead to exaggerated grain growth and decomposition because HA becomes unstable at temperatures exceeding ~ 1300 °C 125, 126, 127, 128, 129, 293,294,295,296. The decomposition temperature of HA is a function of the partial pressure of water vapor. Moreover, processing under vacuum leads to an earlier decomposition of HA, while processing under high partial pressure of water prevents from the decomposition. On the other hand, a presence of water in the sintering atmosphere was reported to inhibit densification of HA and accelerated grain growth 297. Unexpectedly, an application of a magnetic field during sintering was found to influence the growth of HA grains 280. A definite correlation between hardness, density and a grain size in sintered HA bioceramics was found: despite exhibiting high bulk density, hardness started to decrease at a certain critical grain size limit 298, 299, 300.
Since grain growth occurs mainly during the final stage of sintering, to avoid this, a new method called ‘‘two-step sintering’’ (TSS) was proposed 301. The method consists of suppressing grain boundary migration responsible for grain growth, while keeping grain boundary diffusion that promotes densification. The TSS approach was successfully applied to CaPO4 bioceramics 91, 99, 302, 303, 304, 305, 306. For example, HA compacts prepared from nanodimensional powders were two-step sintered. The average grain size of near full dense (> 98 %) HA bioceramics made via conventional sintering was found to be ~ 1.7 μm, while that for TSS HA bioceramics was ~ 190 nm (i.e., ~ 9 times less) with simultaneous increasing the fracture toughness of samples from 0.98 ± 0.12 to 1.92 ± 0.20 MPa m1/2. In addition, due to the lower second step sintering temperature, no HA phase decomposition was detected in TSS method 302.
Hot pressing 300, 307, 308, 309, 310, 311, 312, 313, hot isostatic pressing 100, 200, 205, 207 or hot pressing with post-sintering 314, 315 processes make it possible to decrease a temperature of the densification process, diminish the grain size, as well as achieve higher densities. This leads to finer microstructures, higher thermal stability and subsequently better mechanical properties of CaPO4 scaffolds. Both microwave 316, 317, 318, 319, 320, 321, 322, 323, 324, 325 and spark plasma 82, 116, 326, 327, 328, 329, 330, 331, 332, 333, 334, 335 sintering techniques are alternative methods to the conventional sintering, hot pressing and hot isostatic pressing. Both alternative methods were found to be time and energy efficient densification techniques. Further developments are still possible. For example, a hydrothermal hot pressing method has been developed to fabricate OCP 117, CDHA 336, HA/β-TCP 310 and HA 311, 312, 313, 314 bioceramics with neither thermal dehydration nor thermal decomposition. Further details on the sintering and firing processes of CaPO4 bioceramics are available in literature 127, 338, 339.
To conclude this section, one should mention that the sintering stage is not always necessary. For example, CaPO4 -based scaffolds with the reasonable mechanical properties might be prepared by means of self-setting (self-hardening) formulations 61. Furthermore, the reader’s attention is paid on an excellent review on various ceramic manufacturing techniques 340.
The Major Properties
Mechanical Properties
The modern generation of biomedical materials should stimulate the body’s own self-repairing abilities 341. Therefore, during healing, a mature bone should replace the modern grafts and this process must occur without transient loss of the mechanical support. Unluckily for material scientists, a human body provides one of the most inhospitable environments for the implanted biomaterials. It is warm, wet and both chemically and biologically active. For example, a diversity of body fluids in various tissues might have a solution pH varying from 1 to 9. In addition, a body is capable of generating quite massive force concentrations and the variance in such characteristics among individuals might be enormous. Typically, bones are subjected to ~ 4 MPa loads, whereas tendons and ligaments experience peak stresses in the range of 40 – 80 MPa. The hip joints are subjected to an average load up to three times body weight (3,000 N) and peak loads experienced during jumping can be as high as 10 times body weight. These stresses are repetitive and fluctuating depending on the nature of the activities, which can include standing, sitting, jogging, stretching and climbing. Therefore, all types of implants must sustain attacks of a great variety of aggressive conditions 342. Regrettably, there is presently no artificial material fulfilling all these requirements.
Now it is important to mention, that the mechanical behavior of any ceramics is rather specific. Namely, ceramics is brittle, which is attributed to high strength ionic bonds. Thus, it is not possible for plastic deformation to happen prior to failure, as a slip cannot occur. Therefore, ceramics fail in a dramatic manner. Namely, if a crack is initiated, its progress will not be hindered by the deformation of material ahead of the crack, as would be the case in a ductile material (e.g., a metal). In ceramics, the crack will continue to propagate, rapidly resulting in a catastrophic breakdown. In addition, the mechanical data typically have a considerable amount of scatter 194. Alas, all of these are applicable to CaPO4 bioceramics.
For dense bioceramics, the strength is a function of the grain sizes. Namely, finer grain size bioceramics have smaller flaws at the grain boundaries and thus are stronger than one with larger grain sizes. Thus, in general, the strength for ceramics is proportional to the inverse square root of the grain sizes 343. In addition, the mechanical properties decrease significantly with increasing content of an amorphous phase, microporosity and grain sizes, while a high crystallinity, a low porosity and small grain sizes tend to give a higher stiffness, a higher compressive and tensile strength and a greater fracture toughness. Furthermore, ceramics strength appears to be very sensitive to a slow crack growth 344. Accordingly, from the mechanical point of view, CaPO4 scaffolds appear to be brittle polycrystalline materials for which the mechanical properties are governed by crystallinity, grain size, grain boundaries, porosity and composition 345. Thus, it possesses poor mechanical properties (for instance, a low impact and fracture resistances) that do not allow CaPO4 scaffolds to be used in load-bearing areas, such as artificial teeth or bones. For example, fracture toughness (this is a property, which describes the ability of a material containing a crack to resist fracture and is one of the most important properties of any material for virtually all design applications) of HA bioceramics does not exceed the value of ~ 1.2 MPa·m1/2 346 (human bone: 2 – 12 MPa·m1/2). It decreases exponentially with the porosity increasing 347. Generally, fracture toughness increases with grain size decreasing. However, in some materials, especially non-cubic ceramics, fracture toughness reaches the maximum and rapidly drops with decreasing grain size. For example, a fracture toughness of pure hot pressed HA with grain sizes between 0.2 – 1.2 µm was investigated. The authors found two distinct trends, where fracture toughness decreased with increasing grain size above ~ 0.4 µm and subsequently decreased with decreasing grain size. The maximum fracture toughness measured was 1.20 ± 0.05 MPa·m1/2 at ~ 0.4 µm 307. Fracture energy of HA bioceramics is in the range of 2.3 – 20 J/m2, while the Weibull modulus (it is a measure of the spread or scatter in fracture strength) is low (~ 5 – 12) in wet environments, which means that HA behaves as a typical brittle ceramics and indicates to a low reliability of HA implants 348. Porosity has a great influence on the Weibull modulus 349, 350. In addition, that the reliability of HA scaffolds was found to depend on deformation mode (bending or compression), along with pore size and pore size distribution: a reliability was higher for smaller average pore sizes in bending but lower for smaller pore sizes in compression 351. Interestingly that 3 peaks of internal friction were found at temperatures about –40, 80 and 130 °C for HA but no internal friction peaks were obtained for FA in the measured temperature range; this effect was attributed to the differences of F- and OH- positions in FA and HA, respectively 352. The differences in internal friction values were also found between HA and TCP 353.
Bending, compressive and tensile strengths of dense HA bioceramics are in the ranges of 38 – 250 MPa, 120 – 900 MPa and 38 – 300 MPa, respectively. Similar values for porous HA scaffolds are substantially lower: 2 – 11 MPa, 2 – 100 MPa and ~ 3 MPa, respectively 348. These wide variations in the properties are due to both structural variations (e.g., an influence of remaining microporosity, grain sizes, presence of impurities, etc.) and manufacturing processes, as well as they are caused by a statistical nature of the strength distribution. Strength was found to increase with Ca/P ratio increasing, reaching the maximum value around Ca/P ~ 1.67 (stoichiometric HA) and decreases suddenly when Ca/P > 1.67 348. Furthermore, strength decreases almost exponentially with porosity increasing 354, 355. However, by changing the pore geometry, it is possible to influence the strength of porous bioceramics. It is also worth mentioning that porous CaPO4 scaffolds are considerably less fatigue resistant than dense bioceramics (in materials science, fatigue is the progressive and localized structural damage that occurs when a material is subjected to cyclic loading). Both grain sizes and porosity are reported to influence the fracture path, which itself has a little effect on the fracture toughness of CaPO4 bioceramics 345, 356. However, no obvious decrease in mechanical properties was found after CaPO4 bioceramics had been aged in the various solutions during the different periods of time 357.
Young’s (or elastic) modulus of dense HA bioceramics is in the range of 35 – 120 GPa 358, 359, which is more or less similar to those of the most resistant components of the natural calcified tissues (dental enamel: ~ 74 GPa, dentine: ~ 21 GPa, compact bone: ~ 18 – 22 GPa). This value depends on porosity 360. Nevertheless, dense bulk compacts of HA have mechanical resistances of the order of 100 MPa versus ~ 300 MPa of human bones, diminishing drastically their resistances in the case of porous bulk compacts 361. Young’s modulus measured in bending is between 44 and 88 GPa. To investigate the subject in more details, various types of modeling and calculations are increasingly used 362, 363, 364, 365, 366. For example, the elastic properties of HA appeared to be significantly affected by the presence of vacancies, which softened HA via reducing its elastic modules 366. In addition, a considerable anisotropy in the stress-strain behavior of the perfect HA crystals was found by ab initio calculations 363. The crystals appeared to be brittle for tension along the z-axis with the maximum stress of ~ 9.6 GPa at 10 % strain. Furthermore, the structural analysis of the HA crystal under various stages of tensile strain revealed that the deformation behavior manifested itself mainly in the rotation of PO4 tetrahedrons with concomitant movements of both the columnar and axial Ca ions 363. Data for single crystals are also available 367. Vickers hardness (that is a measure of the resistance to permanent indentation) of dense HA bioceramics is within 3 – 7 GPa, while the Poisson’s ratio (that is the ratio of the contraction or transverse strain to the extension or axial strain) for HA is about 0.27, which is close to that of bones (~ 0.3). At temperatures within 1000 – 1100 °C, dense HA bioceramics was found to exhibit superplasticity with a deformation mechanism based on grain boundary sliding 332, 368, 369. Furthermore, both wear resistance and friction coefficient of dense HA bioceramics are comparable to those of dental enamel 348.
Due to a high brittleness (associated to a low crack resistance), the biomedical applications of CaPO4 bioceramics are focused on production of non-load-bearing implants, such as pieces for middle ear surgery, filling of bone defects in oral or orthopedic surgery, as well as coating of dental implants and metallic prosthesis (see below) 370, 371. Therefore, ways are continuously sought to improve the reliability of CaPO4 bioceramics. Namely, the mechanical properties of sintered bioceramics might be improved by changing the morphology of the initial CaPO4372. In addition, diverse reinforcements (ceramics, metals or polymers) have been applied to manufacture various biocomposites and hybrid biomaterials 373, but that is another story. However, successful hybrid formulations consisted of CaPO4 only 374, 375, 376, 377, 378, 379, 380, 381 are within the scope of this review. Namely, bulk HA bioceramics might be reinforced by HA whiskers 375, 376, 377, 378, 379. Furthermore, various biphasic apatite/TCP formulations were tested 374, 380, 381 and, for example, a superior superplasticity of HA/β-TCP biocomposites to HA bioceramics was detected 380.
Another approach to improve the mechanical properties of CaPO4 bioceramics is to cover the items by polymeric coatings 382, 383, 384 or infiltrate porous structures by polymers 385, 386, 387; however, this is still other story. Further details on the mechanical properties of CaPO4 bioceramics are available elsewhere 347, 348, 388, where the interested readers are referred to.
Porosity
Porosity is defined as a percentage of voids in solids and this morphological property is independent of the material. The surface area of porous bodies is much higher, which guarantees a good mechanical fixation in addition to providing sites on the surface that allow chemical bonding between the bioceramic scaffolds and bones 389. Furthermore, a porous material may have both closed (isolated) pores and open (interconnected) pores. The latter look like tunnels, which are accessible by gases, liquids and particulate suspensions 390. The open-cell nature of porous materials (also known as reticulated materials) is a unique characteristic essential in many applications. In addition, pore dimensions are also important. Namely, the dimensions of open pores are directly related to bone formation, since such pores grant both the surface and space for cell adhesion and bone ingrowth 391, 392, 393. On the other hand, pore interconnection provides the ways for cell distribution and migration, as well as it allows an efficient in vivo blood vessel formation suitable for sustaining bone tissue neo-formation and possibly remodeling 135, 394, 395, 396, 397, 398, 399. Explicitly, porous CaPO4 scaffolds are colonized easily by cells and bone tissues 394, 398, 400, 401, 402, 403, 404, 405, 406, 407. Therefore, interconnecting macroporosity (pore size > 100 μm) 97, 389, 394, 408, 409 is intentionally introduced into solid scaffolds (Figure 5). Calcining of natural bones appears to be the simplest way to prepare porous CaPO4 scaffolds 68, 69, 70, 71, 72, 73, 74. In addition, macroporosity might be formed artificially due to a release of various easily removable compounds and, for that reason, incorporation of pore-creating additives (porogens) is the most popular technique to create macroporosity. The porogens are crystals, particles or fibers of either volatile (they evolve gases at elevated temperatures) or soluble substances. The popular examples comprise paraffin 410, 411, 412, naphthalene 345, 413, 414, 415, sucrose 416, 417, NaHCO3418, 419, 420, NaCl 421, 422, polymethylmethacrylate 87, 423, 424, 425, hydrogen peroxide 426, 427, 428, 429, 430, 431, cellulose derivatives 77. Several other compounds 338, 355, 432, 433, 434, 435, 436, 437, 438, 439, 440, 441, 442, 443 might be used as porogens either. The ideal porogen should be nontoxic and be removed at ambient temperature, thereby allowing the bioceramic/porogen mixture to be injected directly into a defect site and allowing the scaffold to fit the defect 444. Sintering particles, preferably spheres of equal size, is a similar way to generate porous 3D scaffolds of CaPO4. However, pores resulting from this method are often irregular in size and shape and not fully interconnected with one another. Schematic drawings of various types of the ceramic porosity are shown in Figure 6445.
Figure 5.Photographs of a commercially available porous CaPO4 scaffolds with different porosity (top) and a method of their production (bottom). For photos, the horizontal field width is 20 mm.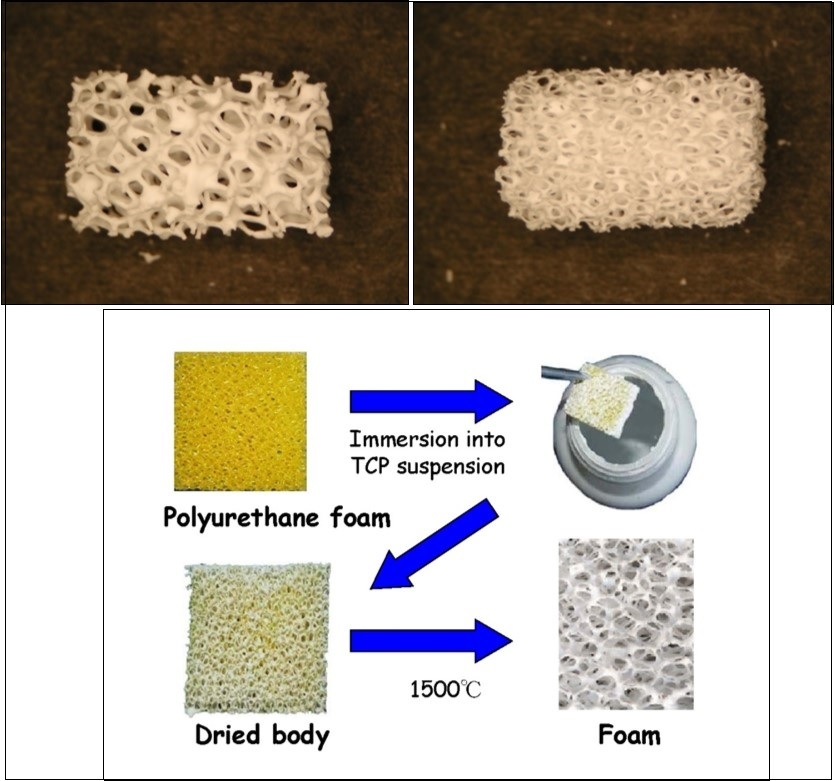
Figure 6.Schematic drawings of various types of the ceramic porosity: A – non-porous, B – microporous, C – macroporous (spherical), D – macroporous (spherical) + micropores, E – macroporous (3D-printing), F – macroporous (3D-printing) + micropores. Reprinted from Ref. 445 with permission.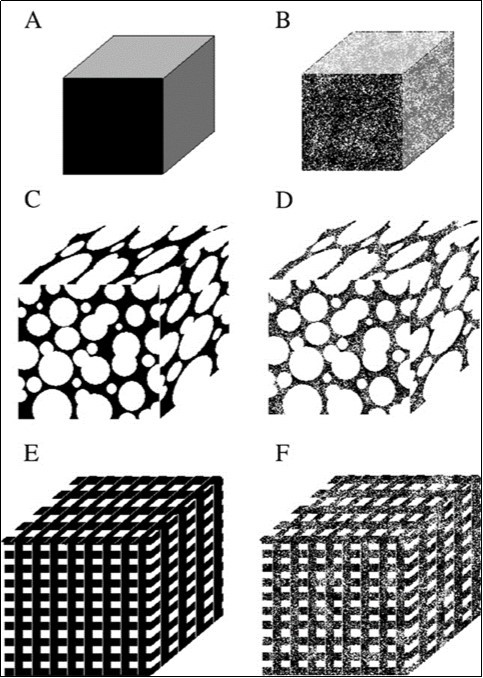
Many other techniques, such as replication of polymer foams by impregnation 236, 237, 238, 446,447,448,449,450 (Figure 5), various types of casting 218, 219, 225, 227, 431, 451, 452, 453, 454, 455, 456, 457, 458, 459, suspension foaming 114, surfactant washing 460, microemulsions 461, 462, ice templating 463, 464, 465, 466, as well as many other approaches 12, 81, 84, 87, 88, 154, 467, 468, 469, 470, 471, 472, 473, 474, 475, 476, 477, 478, 479, 480, 481, 482, 483, 484, 485, 486, 487, 488, 489, 490, 491, 492, 493, 494, 495, 496, 497, 498, 499, 500, 501 have been applied to fabricate porous CaPO4 scaffolds. Some of them have been summarized in Table 3444. In addition, both natural CaCO3 porous materials, such as coral skeletons 502, 503 or shells 503, 504, and artificially prepared ones 505 can be converted into porous CaPO4 under the hydrothermal conditions (250 °C, 24 – 48 h) with the microstructure undamaged. Porous HA scaffolds can also be obtained by hydrothermal hot pressing. This technique allows solidification of the HA powder at 100 – 300 °C (30 MPa, 2 h) 337. In another approach, bi-continuous water-filled microemulsions have been used as pre-organized systems for the fabrication of needle-like frameworks of crystalline HA (2 °C, 3 weeks) 461, 462. Besides, porous CaPO4 might be prepared by a combination of gel casting and foam burn out methods 260, 262, as well as by hardening of the self-setting formulations 411, 412, 419, 420, 422, 432, 433, 490. Lithography was used to print a polymeric material, followed by packing with HA and sintering 471. Hot pressing was applied as well 308, 309. More to the point, a HA suspension can be cast into a porous CaCO3 skeleton, which is then dissolved, leaving a porous network assisted colloidal processing technique 472, 473. In addition, porous HA scaffolds might be prepared by using different starting HA powders and sintering at various temperatures by a pressureless sintering 469. Porous scaffolds with an improved strength might be fabricated from CaPO4 fibers or whiskers. In general, fibrous porous materials are known to exhibit an improved strength due to fiber interlocking, crack deflection and/or pullout 27. Namely, porous scaffolds with well-controlled open pores was processed by sintering of fibrous HA particles 468. In another approach, porosity was achieved by firing apatite-fiber compacts mixed with carbon beads and agar. By varying the compaction pressure, firing temperature and carbon/HA ratio, the total porosity was controlled in the ranges from ~ 40 % to ~ 85 % 77. Finally, a superporous (~ 85 % porosity) HA scaffolds was developed as well 60, 487, 488. Additional information on the processing routes to produce porous ceramics might be found in literature 506, 507.
Table 3. The procedures used to manufacture porous CaPO4 scaffolds for tissue engineering 444.| Year | Location | Process | Apatite from: | Sintering | Compressive strength | Pore size | Porosity | Method of porosity control |
|---|---|---|---|---|---|---|---|---|
| 2006 | Deville et al. Berkeley, CA | HA + ammonium methacrylate in polytetrafluoroethylene mold, freeze dried and sintered | HA #30 | Yes:1300 ºC | 16 MPa65 MPa145 MPa | open unidirectional 50 – 150 μm | > 60 %56 %47 % | Porosity control: slurry conc. Structure controlled by physics of ice front formation. |
| 2006 | Saiz et al. Berkeley, CA | Polymer foams coated, compressed after infiltration, then calcined. | HA powder | Yes: 700 – 1300 ºC | – | 100 – 200 μm | – | Porosity control: extent of compression, HA loading |
| 2006 | Murugan et al. Singapore + USA | Bovine bone cleaned, calcined | bovine bone | Yes: 500 ºC | – | retention of nano-sized pores | – | Porosity control: native porosity of bovine bone |
| 2006 | Xu et al. Gaithersburg, MD | Directly injectable CaPO4 cement, self hardens, mannitol as porogen | nanocrystalline HA | No | 2.2 – 4.2 MPa (flexural) | 0 – 50% macroporous | 65 – 82 % | Porosity control: mannitol mass fraction in mixture |
| 2004 | Landi et al. Italy + Indonesia | Sponge impregnation, isotactic pressing, sintering of HA in simulated body fluid | CaO + H3PO4 | Yes: 1250 ºC for 1 hr | 23 ± 3.8 MPa | closed 6% open 60% | 66 % | Porosity control: possibly by controlling HA particle size. Not suggested by authors |
| 2003 | Charriere et al. EPFL, Switzerland | Thermoplastic negative porosity by Ink jet printing, slip casting process for HA | DCPA + calcite | No: 90 ºC for 1 day | 12.5 ± 4.6 MPa | – | 44 % | Porosity control: negative printing |
| 2003 | Almirall et al. Barcelona, Spain | α-TCP foamed with hydrogen peroxide at different conc., liq. ratios, poured in polytetrafluoroethylene molds | α-TCP + (10% and 20% H2O2) | No: 60 ºC for 2 hr | 1.41 ± 0.27 MPa2.69 ± 0.91 MPa | 35.7% macro 29.7% micro 26.8% macro 33.8% micro | 65.5 % 60.7 % | Porosity control: different concentration, α-TCP particle sizes |
| 2003 | Ramay et al. Seattle, WA | Slurries of HA prepared: gel-casting + polymer sponge technique, sintered. | HA powder | Yes: 600 ºC for1 hr 1350 ºC for 2 hr | 0.5 – 5 MPa | 200 – 400 μm | 70 – 77 % | Porosity control: replicate of polymer sponge template |
| 2003 | Miao et al. Singapore | TTCP to CaPO4 cement. Slurry cast on polymer foam, sintered. | TTCP | Yes: 1200 ºC for 2 hr | – | 1 mm macro5 μm micro | ~ 70% | Porosity control: Recoating time, polyurethane foam |
| 2003 | Uemura et al. China + Japan | Slurry of HA with polyoxyethylene lauryl ether (cross-linked) and sintered | HA powders | Yes: 1200 ºC for 3 hr | 2.25 MPa (0 wk) 4.92MPa(12wks)11.2 MPa (24 wks) | 500 μm 200 μm interconnects | ~ 77 % | Porosity control: polymer interconnects cross-linking |
| 2003 | Ma et al. Singapore + USA | Electrophoretic deposition of HA, sintering. | HA powders | Yes: 1200 ºC for 2 hr | 860 MPa | 0.5 μm130 μm | ~ 20 % | Porosity control: electrophoresis field |
| 2002 | Barralet et al. Birmingham, London, UK | CaPO4 cement + sodium phosphate ice, evaporated | CaCO3 + DCPD | 1st step: 1400 ºC for 1 day | 0.6 ± 0.27 MPa | 2 μm | 62 ± 9 % | Porosity control: porogen shape. |
Scaffold microporosity (pore size < 10 μm), which is defined by its capacity to be impregnated by biological fluids 508, results from the sintering process, while the pore dimensions mainly depend on the material composition, thermal cycle and sintering time. The microporosity provides both a greater surface area for protein adsorption and increased ionic solubility. For example, embedded osteocytes distributed throughout microporous rods might form a mechanosensory network, which would not be possible in scaffolds without microporosity 509, 510. CaPO4 scaffolds with nanodimensional (< 100 nm) pores might be fabricated as well 195, 511, 512, 513, 514, 515. It is important to stress, that differences in porogens usually influence the scaffolds’ macroporosity, while differences in sintering temperatures and conditions affect the percentage of microporosity. Usually, the higher the sintering temperature, the lower both the microporosity content and the specific surface area of the scaffolds. Namely, HA bioceramics sintered at ~ 1200 °C shows significantly less microporosity and a dramatic change in crystal sizes, if compared with that sintered at ~ 1050 °C (Figure 7) 516. Furthermore, the average shape of pores was found to transform from strongly oblate to round at higher sintering temperatures 517. The total porosity (macroporosity + microporosity) of CaPO4 scaffolds was reported to be ~ 70 % 518 or even ~ 85 % 60, 487, 488 of the entire volume. In the case of coralline HA or bovine-derived apatites, the porosity of the original biologic material (coral or bovine bone) is usually preserved during processing 519. To finalize the production topic, creation of the desired porosity in CaPO4 scaffolds is a rather complicated engineering task and the interested readers are referred to the additional publications on the subject 355, 393, 489, 520, 521, 522, 523, 524, 525, 526, 527, 528.
Figure 7.SEM pictures of HA bioceramics sintered at (A) 1050 °C and (B) 1200 °C. Note the presence of microporosity in A and not in B. Reprinted from Ref. 516 with permission.
Regarding the biomedical importance of porosity, studies revealed that increasing of both the specific surface area and pore volume of scaffolds might greatly accelerate the in vivo process of apatite deposition and, therefore, enhance the bone-forming bioactivity. More importantly, a precise control over the porosity, pore dimensions and internal pore architecture of the scaffolds on different length scales is essential for understanding of the structure-bioactivity relationship and the rational design of better bone-forming biomaterials 526, 529, 530. Namely, in antibiotic charging experiments, CaPO4 scaffolds with nanodimensional (< 100 nm) pores showed a much higher charging capacity (1621 μg/g) than that of commercially available CaPO4 (100 μg/g), which did not contain nanodimensional porosity 522. In other experiments, porous blocks of HA were found to be viable carriers with sustained release profiles for drugs 531 and antibiotics over 12 days 532 and 12 weeks 533, respectively. Unfortunately, porosity significantly decreases the strength of implants 348, 356, 388. Thus, porous CaPO4 implants cannot be loaded and are used to fill only small bone defects. However, their strength increases gradually when bones ingrow into the porous network of CaPO4 implants 131, 534, 535, 536, 537. For example, bending strengths of 40 – 60 MPa for porous HA implants filled with 50 – 60 % of cortical bone were reported 534, while in another study an ingrown bone increased strength of porous HA scaffolds by a factor of 3 to 4 536.
Unfortunately, the biomedical effects of scaffolds’ porosity are not straightforward. For example, the in vivo response of CaPO4 of different porosity was investigated and a hardly any effect of macropore dimensions (~ 150, ~ 260, ~ 510 and ~ 1220 μm) was observed 538. In another study, a greater differentiation of mesenchymal stem cells was observed when cultured on ~ 200 μm pore size HA scaffolds when compared to those on ~ 500 μm pore size HA 539. The latter finding was attributed to the fact that a higher pore volume in ~ 500 μm macropore scaffolds might contribute to a lack of cell confluency leading to the cells proliferating before beginning differentiation. Besides, the authors hypothesized that scaffolds with a less than the optimal pore dimensions induced quiescence in differentiated osteoblasts due to reduced cell confluences 539. In still another study, the use of BCP (HA/TCP = 65/35 wt. %) scaffolds with cubic pores of ~ 500 μm resulted in the highest bone formation compared with the scaffold with lower (~ 100 μm) or higher (~ 1000 μm) pore sizes 540. Furthermore, CaPO4 scaffolds with greater strut porosity appeared to be more osteoinductive 541. Already in 1979, Holmes suggested that the optimal pore range was 200 – 400 μm with the average human osteon size of ~ 223 μm 542. In 1997, Tsurga and coworkers implied that the optimal pore size of scaffolds that supported ectopic bone formation was 300 – 400 μm 543. Thus, there is no need to create CaPO4 scaffolds with very big pores; however, the pores must be interconnected 42, 408, 409, 544. Interconnectivity governs a depth of cells or tissue penetration into the porous scaffolds, as well as it allows development of blood vessels required for new bone nourishing and wastes removal 508, 545. Nevertheless, the total porosity of implanted scaffolds appears to be important. For example, 60 % porous β-TCP granules achieved a higher bone fusion rate than 75 % porous β-TCP granules in lumbar posterolateral fusion 509.
Loading by Bioactive Compounds, Drugs and Cells
After being prepared, porous CaPO4 scaffolds are frequently loaded by various types of biomolecules, bioactive compounds, drugs and other therapeutic agents, as well as by genes and/or cells. All of them are added to the scaffolds in hopes that they will match a functionality of the native tissues, provide remodeling to the construct to aid in host integration, and/or be able to spur the host tissue to perform desired actions 546.
Various techniques to incorporate the bioactive compounds and/or cells into pores of the scaffolds, as well as onto the scaffolds’ surface have been reported. The examples comprise blending, surface modification, adsorption, impregnation, centrifugation and vacuum based-techniques 547, 548. Among them, adsorption and impregnation allow these moieties to be incorporated onto the surface of the scaffolds, while centrifugation and vacuum based-techniques enable them to enter into the pores 548. Generally, bioactive compounds, such as growth factors, are incorporated by simple impregnation followed by drying, and the type of bonding with the substrate and the release rate are often undetermined 549. Such associations do not allow chemical bonding between growth factors CaPO4 scaffolds. In such cases, the release rates are difficult to control. For example, precipitation and clustering of the bioactive molecules may occur and the release is only determined by local dissolution and diffusion rules.
An uncontrolled release of bioactive compounds has been related to an accelerated resorption of bone tissue and of the implant. Since bioactive compounds can stimulate the degradation as well as the formation of bone (depending on their local concentrations), they could impair the surface osteoconductivity 550. Namely, bisphosphonates, well established molecules as successful antiresorptive agents for the prevention and treatment of post-menopausal osteoporosis 551, by affecting bone remodeling, could also block the bone repair process: the drug at too high concentration could have detrimental effects on the fixation of the implant over longer periods of time. On the contrary, adsorption leads to stable association and control of the amount of bioactive molecules contained in the solid implant and, thus, of the dose released. Generally, the release is rather low because most of the bioactive molecules adsorbed are irreversibly bound and they are not spontaneously released in a cell culture media 552.
Functionally Graded CaPO4 Scaffolds
Generally, functionally gradient materials (FGMs) are defined as materials, having either compositional or structural gradient from their surface to the interior. The idea of FGMs allows one device to possess two different properties. One of the most important combinations for the biomedical field is that of a mechanical strength and biocompatibility. Namely, only surface properties govern a biocompatibility of the entire device. In contrast, the strongest material determines the mechanical strength of the entire device.
Within the scope of this review, functionally graded CaPO4 scaffolds are considered and discussed only. Such formulations have been developed 87, 455, 458, 524, 553, 554, 555, 556, 557, 558, 559, 560, 561, 562, 563, 564, 565. For example, dense sintered bodies with gradual compositional changes from α-TCP to HA were prepared by sintering a diamond-coated HA compacts at 1280 °C under a reduced pressure, followed by heating under the atmospheric conditions 553. The content of α-TCP gradually decreased, while the content of HA increased with increasing depth from the surface. These functionally gradient scaffolds consisting of HA core and α-TCP surface showed a potential value as bone-substituting biomaterials 553. Two types of functionally gradient FA/β-TCP biocomposites were prepared in another study 554. As shown in Figure 8, one of the graded biocomposites was in the shape of a disk and contained four different layers of about 1 mm thick. The other graded biocomposite was also in the shape of a disk but contained two sets of the four layers, each layer being 0.5 mm thick controlled by using a certain amount of the mixed powders. The final FA/β-TCP graded structures were formed at 100 MPa and sintered at 1300 °C for 2 h 554. The same approach was used in still another study, but HA was used instead of FA and CDHA was used instead of β-TCP 565. CaPO4 coatings with graded crystallinity were prepared as well 560.
Figure 8.A schematic diagram showing the arrangement of the FA/β-TCP biocomposite layers. (a) A non-symmetric functionally gradient material (FGM); (b) symmetric FGM. Reprinted from Ref. 554 with permission.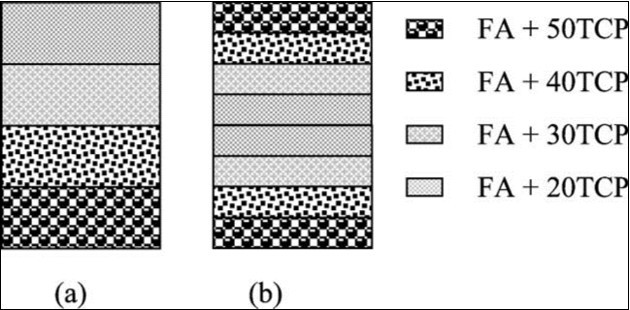
Besides, it is well known that a bone cross-section from cancellous to cortical bone is non-uniform in porosity and pore dimensions. Thus, in various attempts to mimic the porous structure of bones, CaPO4 bioceramics with graded porosity have been fabricated 87, 390, 455, 458, 524, 553, 554, 555, 556, 557, 558. For example, graded porous CaPO4 scaffolds can be produced by means of tape casting and lamination (Figure 9, top). Other manufacturing techniques, such as a compression molding process (Figure 9, bottom) followed by impregnation and firing, are known as well 390. In the first method, a HA slurry was mixed with a pore former. The mixed slurry was then cast into a tape. Using the same method, different tapes with different pore former sizes were prepared individually. The different tape layers were then laminated together. Firing was then done to remove the pore formers and sinter the HA particle compacts, resulting in scaffolds with graded porosity 557. This method was also used to prepare graded porous HA with a dense part (core or layer) in order to improve the mechanical strength, as dense ceramics are much stronger than porous ceramics. However, as in the pressure infiltration of mixed particles, this multiple tape casting also has the problem of poor connectivity of pores, although the pore size and the porosity are relatively easy to control. Furthermore, the lamination step also introduces additional discontinuity of the porosity on the interfaces between the stacked layers.
Figure 9.Schematic illustrations of fabrication of pore-graded bioceramics: top – lamination of individual tapes, manufactured by tape casting; bottom – a compression molding process. Reprinted from Ref. 390 with permission.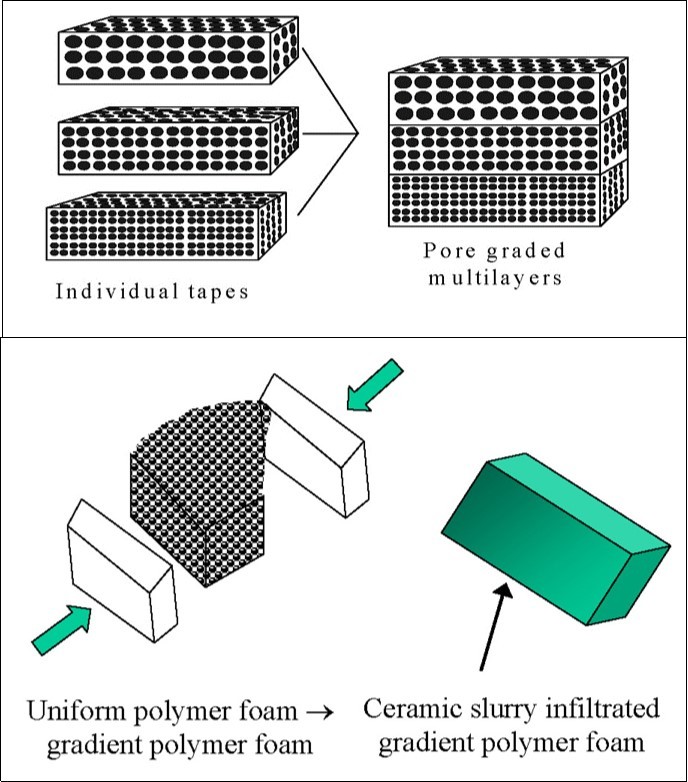
Since diverse biomedical applications require different configurations and shapes, the graded (or gradient) porous scaffolds can be grouped according to both the overall shape and the structural configuration 390. The basic shapes include rectangular blocks and cylinders (or disks). For the cylindrical shape, there are configurations of dense core – porous layer, less porous core – more porous layer, dense layer – porous core and less porous layer – more porous core. For the rectangular shape, in the gradient direction i.e., the direction with varying porosity, pore size or composition, there are configurations of porous top – dense bottom (same as porous bottom – dense top), porous top – dense center – porous bottom, dense top – porous center – dense bottom, etc. Concerning biomedical applications, a dense core – porous layer structure is suitable for implants of a high mechanical strength and with bone ingrowth for stabilization, whereas a less porous layer – more porous core configuration can be used for drug delivery systems. Furthermore, a porous top – dense bottom structure can be shaped into implants of articulate surfaces for wear resistance and with porous ends for bone ingrowth fixation; while a dense top – porous center – dense bottom arrangement mimics the structure of head skull. Further details on scaffolds with graded porosity might be found in literature 390.
Biological Properties and the in Vivo Behavior
The most important differences between bioactive scaffolds and all other implanted materials comprise inclusion in the metabolic processes of the organism, adaptation of either surface or the entire material to the biomedium, integration of a bioactive implant with bone tissues at the molecular level or the complete replacement of a resorbable bioceramics by healthy bone tissues. All of the enumerated processes are related to the effect of an organism on the implant. Nevertheless, another aspect of implantation is also important – the effect of the implant on the organism. For example, using of bone implants from corpses or animals, even after they have been treated in various ways, provokes a substantially negative immune reactions in the organism, which substantially limits the application of such implants. In this connection, it is useful to dwell on the biological properties of bioceramic scaffolds, particularly those of CaPO4, which in the course of time may be resorbed completely 566.
Interactions with the Surrounding Tissues and the Host Responses
All interactions between implants and the surrounding tissues are dynamic processes. Water, dissolved ions, various biomolecules and cells surround the implant surface within initial few seconds after the implantation. It has been accepted that no foreign material placed inside a living body is completely compatible. The only substances that conform completely are those manufactured by the body itself (autogenous), while any other substance, which is recognized as foreign, initiates some types of reactions (a host-tissue response). The reactions occurring at the biomaterial/tissue interfaces lead to time-dependent changes in the surface characteristics of both the implanted biomaterials and the surrounding tissues 567.
In order to develop new scaffolds, it is necessary to understand the in vivo host responses. Like any other species, biomaterials and bioceramics react chemically with their environment and, ideally, they should neither induce any changes nor provoke undesired reactions in the neighboring or distant tissues. In general, living organisms can treat artificial implants as biotoxic (or bioincompatible 568), bioinert (or biostable 59), biotolerant (or biocompatible 568), bioactive and bioresorbable materials 566, 567, 568, 569. Biotoxic (e.g., alloys containing cadmium, vanadium, lead and other toxic elements) materials release to the body substances in toxic concentrations and/or trigger the formation of antigens that may cause immune reactions ranging from simple allergies to inflammation to septic rejection with the associated severe health consequences. They cause atrophy, pathological change or rejection of living tissue near the material as a result of chemical, galvanic or other processes. Bioinert (this term should be used with care, since it is clear that any material introduced into the physiological environment will induce a response. However, for the purposes of biomedical implants, the term can be defined as a minimal level of response from the host tissue), such as zirconia, alumina, carbon and titanium, as well as biotolerant (e.g., polymethylmethacrylate, titanium and Co-Cr alloy) materials do not release any toxic constituents but also do not show positive interaction with living tissue. They evoke a physiological response to form a fibrous capsule, thus, isolating the material from the body. In such cases, thickness of the layer of fibrous tissue separating the material from other tissues of an organism can serve as a measure of bioinertness. Generally, both bioactivity and bioresorbability phenomena are fine examples of chemical reactivity and CaPO4 (both non-substituted and ion-substituted ones) fall into these two categories of bioceramics 566, 567, 568, 569. A bioactive material will dissolve slightly but promote formation of a surface layer of biological apatite before interfacing directly with the tissue at the atomic level, that result in formation of a direct chemical bonds to bones. Such implants provide a good stabilization for materials that are subject to mechanical loading. A bioresorbable material will dissolve over time (regardless of the mechanism leading to the material removal) and allow a newly formed tissue to grow into any surface irregularities but may not necessarily interface directly with the material. Consequently, the functions of bioresorbable materials are to participate in dynamic processes of formation and re-absorption occurring in bone tissues; thus, bioresorbable materials are used as scaffolds or filling spacers allowing to the tissues their infiltration and substitution 193, 338, 570, 571, 572.
It is important to stress, that a distinction between the bioactive and bioresorbable bioceramics might be associated with structural factors only. Namely, bioceramics made from non-porous, dense and highly crystalline HA behaves as a bioinert (but a bioactive) material and is retained in an organism for at least 5 – 7 years without noticeable changes (Figure 3 bottom), while a highly porous bioceramics of the same composition can be resorbed approximately within a year. Furthermore, submicron-sized HA powders are biodegraded even faster than the highly porous HA scaffolds. Other examples of bioresorbable materials comprise porous bioceramic scaffolds made of biphasic, triphasic or multiphasic CaPO4 formulations 92 or bone grafts (dense or porous) made of CDHA 133, TCP 87, 573, 574 and/or ACP 434, 575. One must stress that at the beginning of 2000-s the concepts of bioactive and bioresorbable materials have been converged and bioactive materials are made bioresorbable, while bioresorbable ones are made bioactive 576.
Although in certain in vivo experiments inflammatory reactions were observed after implantation or injection of CaPO4577, 578, 579, 580, 581, 582, 583, 584, 585, 586, the general conclusion on using CaPO4 with Ca/P ionic ratio within 1.0 – 1.7 is that all types of implants (scaffolds of various porosities and structures, as well as, powders or granules) are not only nontoxic but also induce neither inflammatory nor foreign-body reactions 120, 587, 588. The biological response to implanted CaPO4 scaffolds follows a similar cascade observed in fracture healing. This cascade includes a hematoma formation, inflammation, neovascularization, osteoclastic resorption and a new bone formation. An intermediate layer of fibrous tissue between the implants and bones has been never detected. Furthermore, CaPO4 implants display the ability to directly bond to bones 566, 567, 568, 569. For further details, the interested readers are referred to a good review on cellular perspectives of bioceramic scaffolds for bone tissue engineering 444.
One should note that the aforementioned rare cases of the inflammatory reactions to CaPO4 scaffolds were often caused by “other” reasons. For example, a high rate of wound inflammation occurred when highly porous HA scaffolds were used. In that particular case, the inflammation was explained by sharp implant edges, which irritated surrounding soft tissues 578. To avoid this, only rounded material should be used for implantation (Figure 10) 589. Another reason for inflammation produced by HA scaffolds could be due to micro movements of the implants, leading to simultaneous disruption of a large number of micro-vessels, which grow into the pores of scaffolds. This would immediately produce an inflammatory reaction. Additionally, problems could arise in clinical tests connected with migration of granules used for alveolar ridge augmentation, because it might be difficult to achieve a mechanical stability of implants at the implantation sites 578. Besides, presence of calcium pyrophosphate impurity might be the reason of inflammation 581. Additional details on inflammatory cell responses to CaPO4 might be found in a special review on this topic 582.
Figure 10.Rounded β-TCP granules of 2.6 – 4.8 mm in size, providing no sharp edges for combination with bone cement. Reprinted from Ref. 589 with permission.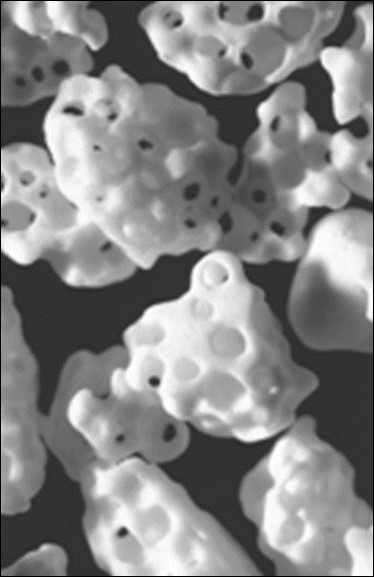
Osteoinduction
Before recently, it was generally considered, that alone, any type of synthetic scaffolds possessed neither osteogenic (osteogenesis is the process of laying down new bone material by osteoblasts 590) nor osteoinductive (is the property of the material to induce bone formation de novo or ectopically (i.e., in non-bone forming sites) 590) properties and demonstrated a minimal immediate structural support. However, a number of reports have already shown the osteoinductive properties of certain types of CaPO4 bioceramics 164, 516, 541, 591, 592, 593, 594, 595, 596, 597, 598, 599, 600, 601, 602, 603, 604, 605, 606, 607, 608, 609, 610 and the amount of such publications rapidly increases. For example, bone formation was found to occur in dog muscle inside porous CaPO4 scaffolds with surface microporosity, while bone was not observed on the surface of dense bioceramics 595. Furthermore, implantation of porous β-TCP scaffolds appeared to induce bone formation in soft tissues of dogs, while no bone formation was detected in any α-TCP ones 592. More to the point, titanium implants coated by a microporous layer of OCP were found to induce ectopic bone formation in goat muscles, while a smooth layer of carbonated apatite on the same implants was not able to induce bone formation there 593, 594. In another study, β-TCP powder, biphasic (HA + β-TCP) powder and intact biphasic (HA + β-TCP) rods were implanted into leg muscles of mice and dorsal muscles of rabbits 601. One month and three months after implantation, samples were harvested for biological and histological analysis. New bone tissues were observed in 10 of 10 samples for β-TCP powder, 3 of 10 samples biphasic powder and 9 of 10 samples for intact biphasic rods at 3rd month in mice, but not in rabbits. The authors concluded that the chemical composition was the prerequisite in osteoinduction, while porosity contributed to more bone formation 601. Therefore, researchers have already discovered the ways to prepare osteoinductive CaPO4 scaffolds.
Unfortunately, the underlying mechanism(s) leading to bone induction by synthetic materials remains largely unknown. Nevertheless, besides the specific genetic factors 599 and chosen animals 601, the dissolution/precipitation behavior of CaPO4611, their particle size 609, microporosity 597, 601, 612, 613, physicochemical properties 595, 597, composition 601, the specific surface area 613, nanostructure 600, as well as the surface topography and geometry 596, 614, 615, 616, 617, 618 have been pointed out as the relevant parameters. A positive effect of increased microporosity on the ectopic bone formation could be both direct and indirect. Firstly, an increased microporosity is directly related to the changes in surface topography, i.e. increases a surface roughness, which affects the cellular differentiation 618. Secondly, an increased microporosity indirectly means a larger surface that is exposed to the body fluids leading to elevated dissolution/precipitation phenomena as compared to non-microporous surfaces. In addition, other hypotheses are also available. Namely, Reddi explained the apparent osteoinductive properties as an ability of particular bioceramics to concentrate bone growth factors, which are circulating in biological fluids, and those growth factors induce bone formation 614. Other researchers proposed a similar hypothesis that the intrinsic osteoinduction by CaPO4 scaffolds was a result of adsorption of osteoinductive substances on their surface 596. Moreover, Ripamonti 615 and Kuboki et al., 616 independently postulated that the geometry of CaPO4 bioceramics is a critical parameter in bone induction. Specifically, bone induction by CaPO4 was never observed on flat surfaces. All osteoinductive cases were observed on either porous scaffolds or structures contained well-defined concavities. What’s more, bone formation was never observed on the peripheries of porous scaffolds and was always found inside the pores or concavities, aligning the surface 193. Some researchers speculated that a low oxygen tension in the central region of implants might provoke a dedifferentiation of pericytes from blood micro-vessels into osteoblasts 619. Finally but yet importantly, both nano-structured rough surfaces and a surface charge on implants were found to cause an asymmetrical division of the stem cells into osteoblasts, which is important for osteoinduction 612.
Nevertheless, to finalize this topic, it is worth citing a conclusion made by Boyan and Schwartz 620: “Synthetic materials are presently used routinely as osteoconductive bone graft substitutes, but before purely synthetic materials can be used to treat bone defects in humans where an osteoinductive agent is required, a more complete appreciation of the biology of bone regeneration is needed. An understanding is needed of how synthetic materials modulate the migration, attachment, proliferation and differentiation of mesenchymal stem cells, how cells on the surface of a material affect other progenitor cells in the peri-implant tissue, how vascular progenitors can be recruited and a neovasculature maintained, and how remodeling of newly formed bone can be controlled.” (p. 9).
Biodegradation
Shortly after implantation, a healing process is initiated by compositional changes of the surrounding bio-fluids and adsorption of biomolecules. Following this, various types of cells reach the surface of CaPO4 scaffolds and the adsorbed layer dictates the ways the cells respond. Further, a biodegradation (which can be envisioned as an in vivo process by which an implanted material breaks down into either simpler components or components of the smaller dimensions) of the implanted CaPO4 scaffolds begin. This process can occur by three possible ways: 1) physical: due to abrasion, fracture and/or disintegration, 2) chemical: due to physicochemical dissolution of the implanted phases of CaPO4 with a possibility of phase transformations into other phases of CaPO4, as well as their precipitation and 3) biological: due to cellular activity (so called, bioresorption). In biological systems, all these processes take place simultaneously and/or in competition with each other. Since the existing CaPO4 are differentiated by Ca/P ratio, basicity/acidity and solubility (Table 1), in the first instance, their degradation kinetics and mechanisms depend on the chosen type of CaPO4621, 622. Since dissolution is a physical chemistry process, it is controlled by some factors, such as CaPO4 solubility, surface area to volume ratio, local acidity, fluid convection and temperature. For HA and FA, the dissolution mechanism in acids has been described by a sequence of four successive chemical equations, in which several other CaPO4, such as TCP, DCPD/DCPA and MCPM/MCPA, appear as virtual intermediate phases 623, 624.
With a few exceptions, dissolution rates of CaPO4 are inversely proportional to the Ca/P ratio (except of TTCP), phase purity and crystalline size, as well as it is directly related to both the porosity and the surface area. In addition, phase transformations might occur with DCPA, DCPD, OCP, α-TCP, β-TCP and ACP because they are unstable in aqueous environment under the physiological conditions 625. Bioresorption is a biological process mediated by cells (mainly, osteoclasts and, in a lesser extent, macrophages) 626, 627. It depends on the response of cells to their environment. Osteoclasts attach firmly to the implant and dissolve CaPO4 by secreting an enzyme carbonic anhydrase or any other acid, leading to a local pH drop to ~ 4 – 5 628. Formation of multiple spine-like crystals at the exposed areas of β-TCP was discovered 629. Furthermore, nanodimensional particles of CaPO4 can also be phagocytosed by cells, i.e. they are incorporated into cytoplasm and thereafter dissolved by acid attack and/or enzymatic processes 630. A study is available 631, in which a comparison was made between the solubility and osteoclastic resorbability of 3 types of CaPO4 (DCPA, ACP and HA) + β-calcium pyrophosphate (β-CPP) powders having the monodisperse particle size distributions. The authors discovered that with the exception of β-CPP, the difference in solubility among different calcium phosphates became neither mitigated nor reversed but augmented in the resorptive osteoclastic milieu. Namely, DCPA (the phase with the highest solubility) was resorbed more intensely than any other calcium phosphate, whereas HA (the phase with the lowest solubility) was resorbed the least. β-CPP became retained inside the cells for the longest period of time, indicating hindered digestion of only this particular type of calcium phosphate. Genesis of osteoclasts was found to be mildly hindered in the presence of HA, ACP and DCPA, but not in the presence of β-CPP. HA appeared to be the most viable compound with respect to the mitochondrial succinic dehydrogenase activity. The authors concluded that chemistry did have a direct effect on biology, while biology neither overrode nor reversed the chemical propensities of calcium phosphates with which it interacted, but rather augmented and took a direct advantage of them 631. Similar conclusions on both the resorbability and dissolution behavior of OCP, β-TCP and HA were made in another study 625. In addition, in vivo biodegradation of MCPA was found to be faster than that of bovine HA 632. Thus, one can conclude that in vivo biodegradation kinetics of CaPO4 seems to correlate well with their solubility. Nevertheless, one must keep in mind that this is a very complicated combination of various non-equilibrium processes, occurring simultaneously and/or in competition with each other 633.
Strictly speaking, the processes happen in vitro do not necessarily represent the ones occurring in vivo and vice versa; nevertheless, in vitro experiments are widely performed. Usually, an in vitro biodegradation of CaPO4 scaffolds is simulated by suspending them in a slightly acidic (pH ~ 4) buffer and monitoring the release of major ions with time 622, 634, 635, 636, 637. An acidic buffer, to some extent, mimics the acidic environment during osteoclastic activity. In one study, an in vivo behavior of porous β-TCP scaffolds prepared from rod-shaped particles and that prepared from non-rod-shaped particles in the rabbit femur was compared. Although the porosities of both types of β-TCP scaffolds were almost the same, a more active osteogenesis was preserved in the region where rod-shaped ones were implanted 638. Furthermore, the dimensions of both the particles 609 and the surface microstructure 604 were found to influence the osteoinductive potential. These results implied that the microstructure affected the activity of bone cells and subsequent bone replacement.
Cellular Response
Fixation of any implants in the body is a complex dynamic process that remodels the interface between the implants and living tissues at all dimensional levels, from the molecular up to the cell and tissue morphology level, and at all time scales, from the first second up to several years after implantation. Immediately following the implantation, a space filled with biological fluids appears next to the implant surface. With time, cells are adsorbed at the implant surface that will give rise to their proliferation and differentiation towards bone cells, followed by revascularisation and eventual gap closing. Ideally, a strong bond is formed between the implants and surrounding tissues 568. An interesting study on the interfacial interactions between calcined HA and substrates has been performed 639, where the interested readers are referred for further details.
The aforementioned paragraph clearly demonstrates an importance of studies on cellular responses to CaPO4 scaffolds. Therefore, such investigations have been performed extensively for several decades 582, 640, 641, 642, 643, 644, 645, 646, 647, 648, 649, 650, 651, 652, 653, 654, 655. For example, bioceramic discs made of 7 different types of CaPO4 (TTCP, HA, carbonate apatite, β-TCP, α-TCP, OCP and DCPD) were incubated in osteoclastic cell cultures for 2 days. In all cases, similar cell morphologies and good cell viability were observed; hoverer, different levels of resorbability of various types of CaPO4 were detected 643. Similar results were found for fluoridated HA coatings 645. Experiments performed with human osteoblasts revealed that nanostructured bioceramics prepared from nano-sized HA showed significant enhancement in mineralization compared to microstructured HA bioceramics 644. In addition, the influence of lengths and surface areas of rod-shaped HA on cellular response were studied. Again, similar cell morphologies and good cell viability were observed; however, it was concluded that high surface area could increase cell-particle interaction 648. Nevertheless, another study with cellular response to rod-shaped HA bioceramics, revealed that some types of crystals might trigger a severe inflammatory response 651. In addition, CaPO4-based sealers appeared to show less cytotoxicity and inflammatory mediators compared with other sealers 646. More examples are available in literature.
Cellular biodegradation of CaPO4 scaffolds is known to depend on its phases. For example, a higher solubility of β-TCP was shown to prevent L-929 fibroblast cell adhesion, thereby leading to damage and rupture of the cells 656. A mouse ectopic model study indicated the maximal bone growth for the 80 : 20 β-TCP : HA biphasic formulations preloaded with human mesenchymal stem cells when compared to other CaPO4657. The effects of substrate microstructure and crystallinity have been corroborated with an in vivo rabbit femur model, where rod-like crystalline β-TCP was reported to enhance osteogenesis when compared to non-rod like crystalline β-TCP 638. Additionally, using a dog mandibular defect model, a higher bone formation on a scaffold surface coated by nanodimensional HA was observed when compared to that coated by a micro-dimensional HA 658. Furthermore, studies revealed a stronger stress signaling response by osteoblast precursor cells in 3D scaffolds when compared to 2D surfaces 659.
Mesenchymal stem cells are one of the most attractive cellular lines for application as bone grafts 660, 661. Early investigations by Okumura, et al. indicated an adhesion, proliferation and differentiation, which ultimately became new bone and integrated with HA scaffolds 641. Later, a sustained co-culture of endothelial cells and osteoblasts on HA scaffolds for up to 6 weeks was demonstrated 662. Furthermore, a release of factors by endothelial and osteoblast cells in co-culture supported proliferation and differentiation was suggested to ultimately result in microcapillary-like vessel formation and supported a neo-tissue growth within the scaffold 444. More to the point, investigation of rat calvaria osteoblasts cultured on transparent HA bioceramics, as well as the analysis of osteogenic-induced human bone marrow stromal cells at different time points of culturing indicated to a good cytocompatibility of HA bioceramics and revealed favorable cell proliferation. The positive results for other types of cells have been obtained in other studies 202, 403,404,405, 663,664,665.
Interestingly that HA scaffolds with marrow stromal cells in a perfused environment were reported to result in ~ 85 % increase in mean core strength, a ~ 130 % increase in failure energy and a ~ 355 % increase in post-failure strength. The increase in mineral quantity and promotion of the uniform mineral distribution in that study was suggested to attribute to the perfusion effect 535. Additionally, other investigators indicated to mechanical properties increasing for other CaPO4 scaffolds after induced osteogenesis 534, 537.
To finalize this section, one should mention on the recent developments to influence the cellular response. First, to facilitate interactions with cells, the surface of CaPO4 scaffolds can be functionalized 666, 667, 668, 669, 670. Second, it appears that crystals of biological apatite of calcified tissues exhibit different orientations depending on the tissue. Namely, in vertebrate bones and tooth enamel surfaces, the respective a, b-planes and c-planes of the apatite crystals are preferentially exposed. Therefore, ideally, this should be taken into account in artificial bone grafts. Recently, a novel process to fabricate dense HA bioceramics with highly preferred orientation to the a,b-plane was developed.
The results revealed that increasing the a,b-plane orientation degree shifted the surface charge from negative to positive and decreased the surface wettability with simultaneous decreasing of cell attachment efficiency
671,672,673. The latter finding resulted in further developments on preparation of oriented CaPO4 compounds 674, 675, 676.
Future Developments
Philosophically, the increase in life expectancy requires biological solutions to all biomedical problems, including orthopedic ones, which previously were managed with mechanical solutions. Therefore, since the end of 1990’s, the biomaterials research focuses on tissue regeneration instead of tissue replacement 677.
The alternatives include use hierarchical bioactive scaffolds to engineer in vitro living cellular constructs for transplantation or use bioresorbable bioactive particulates or porous networks to activate in vivo the mechanisms of tissue regeneration 678, 679. Thus, the aim of CaPO4 is to prepare artificial porous scaffolds able to provide the physical and chemical cues to guide cell seeding, differentiation and assembly into 3D tissues of a newly formed bone. Particle sizes, shape and surface roughness of the scaffolds are known to affect cellular adhesion, proliferation and phenotype 33, 34, 35, 36, 37, 38, 39. Additionally, the surface energy might play a role in attracting particular proteins to the CaPO4 surface and, in turn, this will affect the cells affinity to the material. More to the point, cells are exceedingly sensitive to the chemical composition and their bone-forming functions can be dependent on grain morphology of the scaffolds. For example, osteoblast functions were found to increase on nanodimensional fibers if compared to nanodimensional spheres because the former more closely approximated the shape of biological apatite in bones 680. Besides, a significantly higher osteoblast proliferation on HA bioceramics sintered at 1200 °C as compared to that on HA bioceramics sintered at 800 °C and 1000 °C was reported 681. Furthermore, since ions of calcium and orthophosphate are known to regulate bone metabolism, CaPO4 appear to be among the few bone graft substitute materials, which can be considered as a drug. A schematic drawing of the key scaffold properties affecting a cascade of biological processes occurring after CaPO4 implantation is shown in Figure 11682.
Figure 11.A schematic drawing of the key scaffold properties affecting a cascade of biological processes occurring after CaPO4 implantation. Reprinted from Ref. 682 with permission.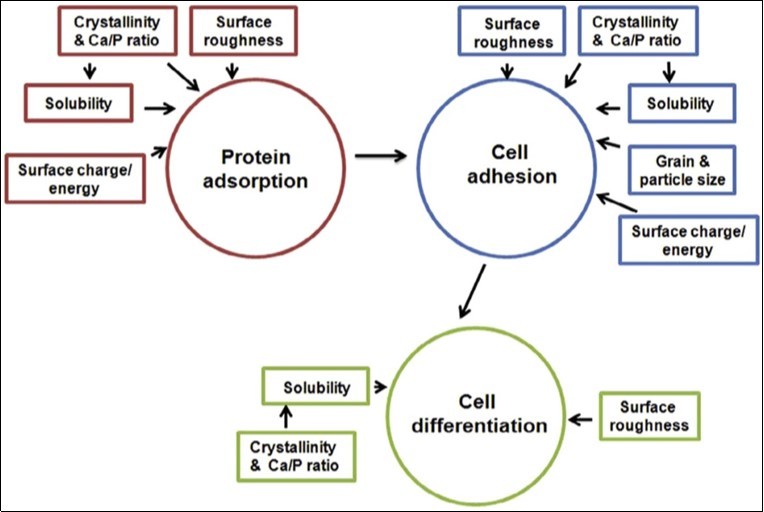
Thus, to meet the tissue engineering requirements, much attention is devoted to further improvements of CaPO4 scaffolds 683, 684, 685. From the chemical point of view, the developments include synthesis of novel ion-substituted CaPO4686, 687, 688, 689, 690. From the material point of view, the major research topics include nanodimensional and nanocrystalline structures 691, 692, 693, 694, amorphous compounds 695, 696, (bio)organic/CaPO4 biocomposites and hybrid formulations 373, 697, 698, biphasic, triphasic and multiphasic formulations 92, as well as various types of structures, forms and shapes. The latter comprise fibers, whiskers and filaments 699, 700, 701, 702, 703, 704, 705, 706, 707, 708, 709, 710, 711, 712, macro-, micro- and nano-sized spheres, beads and granules 711, 712, 713, 714, 715, 716, 717, 718, 719, 720, 721, 722, 723, 724, 725, 726, 727, 728, 729, 730, 731, micro- and nano-sized tubes 732, 733, 734, 735, 736, porous 3D scaffolds made of ACP 737, TCP 81, 84, 738, 739, 740, 741, HA 56, 742, 743, 744, 745, 746 and biphasic formulations 716, 728, 741, 747, 748, 749, 750, 751, 752, structures with graded porosity 87, 390, 455, 458, 524, 553, 554, 555, 556, 557, 558 and hierarchically organized ones 753, 754. Furthermore, an addition of defects through an intensive milling 755, 756 or their removal by a thermal treatment 757 can be used to modify a chemical reactivity of CaPO4. Besides, more attention should be paid to a crystallographically aligned CaPO4 bioceramics 758.
Clinical Applications
To date, there are just a few publications on clinical application of cell-seeded CaPO4 scaffolds for bone tissue engineering of humans. Namely, Quarto et al., 759 were the first to report a treatment of large (4 – 7 cm) bone defects of the tibia, ulna and humerus in three patients from 16 to 41 years old, where the conventional surgical therapies had failed. The authors implanted a custom-made unresorbable porous HA scaffolds seeded with in vitro expanded autologous bone marrow stromal cells. In all three patients, radiographs and computed tomographic scans revealed abundant callus formation along the implants and good integration at the interfaces with the host bones by the second month after surgery 759. In the same year, Vacanti et al., 760 reported the case of a man who had a traumatic avulsion of the distal phalanx of a thumb. The phalanx was replaced with a specially treated natural coral (porous HA; 500-pore ProOsteon) implant that was previously seeded with in vitro expanded autologous periosteal cells. The procedure resulted in the functional restoration of a stable and biomechanically sound thumb of normal length, without the pain and complications that are usually associated with harvesting a bone graft.
Morishita et al., 761 treated a defect resulting from surgery of benign bone tumors in three patients using HA scaffolds seeded with in vitro expanded autologous bone marrow stromal cells after osteogenic differentiation of the cells. Two bone defects in a tibia and one defect in a femur were treated. Although ectopic implants in nude mice were mentioned to show the osteogenicity of the cells, details such as the percentage of the implants containing bone and at what quantities were not reported. Furthermore, cell-seeded CaPO4 scaffolds were found to be superior to autograft, allograft or cell-seeded allograft in terms of bone formation at ectopic implantation sites 762.
An innovative appliance named the stem cell screen-enrich-combine(-biomaterials) circulating system (SECCS) was designed in another study 763. In that study, 42 patients who required bone graft underwent SECCS-based treatment. Their bone marrow samples and β-TCP granules were processed in the SECCS for 10-15 minutes, to produce MSC/β-TCP composites. These composites were grafted back into bone defect sites. The results showed 85.53% ± 7.95% autologous mesenchymal stem cells were successfully screened, enriched, and seeded on the β-TCP scaffolds synchronously. Clinically, all patients obtained satisfactory bone healing 763.
Besides, it has been hypothesized that dental follicle cells combined with β-TCP scaffolds might become a novel therapeutic strategy to restore periodontal defects 764. In still another study, the behavior of human periodontal ligament stem cells on a HA-coated genipin-chitosan scaffold in vitro was studied followed by evaluation on bone repair in vivo 765. The study demonstrated the potential of this formulation for bone regeneration.
To finalize this section, one must mention that CaPO4 scaffolds are also used in veterinary orthopedics for favoring animal bone healing in areas, in which bony defects exist 766, 767.
Conclusions
The field of bone tissue engineering has experienced exponential growth over the last 30 years, however the disconnect between research and the clinic, with a pull towards complexity in academia and a push back towards simplicity and in the clinical setting, looms to hinder further progress in the field. From a clinical point of view, bone defects and fractures vary in its specific cause e.g., fracture (single- vs. multi-fragmentary), non-unions, tumor, etc. and each patient has an naturally personalized endogenous healing pattern based on their physical status, age, relevant comorbidities, compliance and loading patterns, and life style choices such as smoking, diet, etc. Therefore, a “one concept fits all” solution is unlikely to be successful in any therapy concept rooted in regenerative medicine. In addition, researchers also need to take into account the end user of the products, namely the surgeons. Over complicated engineering designs can hinder the overall adoption rate of new technologies and treatment options, emphasis should be placed on product usability and technical feasibility. Moreover, due to the innate regenerative potential of bones, researchers should place less emphasis on precisely mimicking the physical properties of the tissue to be regenerated and more on facilitating and leverage the guidance of the endogenous regenerative capabilities of the host tissues 768.
To conclude, one should mention that despite the remarkable scientific progress over the last decades, we are still far away from “pulling a newly engineered organ out of the Petri dish”. Therefore, a variety of scaffolds have been already developed for bone tissue engineering to be used as spacemakers, biodegradable substitutes for transplanting to the new bones, matrices for drug delivery system, as well as supporting structures enhancing adhesion, proliferation and production of seeded cells according to the circumstances of the bone defects. Nevertheless, scaffolds to be clinically completely satisfied have not been developed yet. Therefore, development of more functional scaffolds is required 768.
References
- 1.Seeman E, P D Delmas. (2006) Bone quality – the material and structural basis of bone strength and fragility. N EnglJMed. 354-2250.
- 2.P V Giannoudis, Dinopoulos H, Tsiridis E. (2005) Bone substitutes: an update. Injury,36,Suppl.3,S20-S27
- 3.M P Lutolf, J A Hubbell. (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. , Nat. Biotechnol 23, 47-55.
- 4.J R Wolter, R F Meyer. (1984) Sessile macrophages forming clear endothelium-like membrane on inside of successful keratoprosthesis. , Trans. Am. Ophthalmol. Soc 82, 187-202.
- 5.Skalak R. (1988) Fox C F.(Eds.) Tissue engineering. Proceedings of a workshop held at Granlibakken. , Lake Tahoe, CA. New York, NY: Liss; 26-29.
- 10.Bose S, Tarafder S. (2012) Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 8, 1401-1421.
- 13.J R, L.Regeneration of trabecular bone using porous ceramics. , Curr. Opin. Solid State Mater. Sci 2003, 301-307.
- 14.Vallet-Regí M, J M González-Calbet. (2004) Calcium phosphates as substitution of bone tissues. Progr. Solid State Chem. 32, 1-31.
- 15.Schlickewie W, Schlickewie C. (2007) The use of bone substitutes in the treatment of bone defects – the clinical view and history. Macromol. Symp 253-10.
- 16.S V Dorozhkin. (2012) Calcium orthophosphates: applications in nature, biology, and medicine. , Pan Stanford: Singapore 850.
- 17.S V Dorozhkin. (2016) Calcium orthophosphate-based bioceramics and biocomposites. , Wiley-VCH: Weinheim, Germany 405.
- 18.P V Giannoudis, T A Einhorn, Marsh D. (2007) Fracture healing: the diamond concept. Injury. 38, 3-6.
- 19.P V Giannoudis, T A Einhorn, Schmidmaier G, Marsh D. (2008) The diamond concept – open questions. Injury. 39, 5-8.
- 21.D W Hutmacher. (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials. 21, 2529-2543.
- 23.El-Ghannam A. (2005) Bone reconstruction: from bioceramics to tissue engineering. , Expert Rev. Med. Dev 2, 87-101.
- 24.Kneser U, D J Schaefer, Polykandriotis E, R E Horch. (2006) Tissue engineering of bone: the reconstructive surgeon’s point of view. , J. Cell. Mol. Med 10, 7-19.
- 25.T G Scott, Blackburn G, Ashley M, I S Bayer, Ghosh A et al.Advances in bionanomaterials for bone tissue engineering. , J. Nanosci. Nanotechnol 2013, 1-22.
- 27.Moroni L, Wijn J R de, Blitterswijk C A van. (2008) Integrating novel technologies to fabricate smart scaffolds. , J. Biomater. Sci. Polymer Edn 19, 543-572.
- 28.Yasuhiko T. (2009) Biomaterial technology for tissue engineering applications. , J. R. Soc. Interface 6, 311-324.
- 30.Schieker M, Seitz H, Drosse I, Seitz S, Mutschler W. (2006) Biomaterials as scaffold for bone tissue engineering. , Eur. J. Trauma 32, 114-124.
- 31.D F Williams. (2014) The biomaterials conundrum in tissue engineering. Tissue Eng. A. 20, 1129-1131.
- 32.L E Freed, Guilak F, X E Guo, M L Gray, Tranquillo R et al. (2006) Advanced tools for tissue engineering: Scaffolds, bioreactors, and signaling. Tissue Eng. 12, 3285-3305.
- 33.Gandaglia A, Bagno A, Naso F, Spina M, G Cells.scaffolds and bioreactors for tissue-engineered heart valves: a journey from basic concepts to contemporary developmental innovations. , Eur. J. Cardiothorac. Surg 2011, 523-531.
- 34.Hui J H P, K S Buhary, Chowdhary A. (2012) Implantation of orthobiologic, biodegradable scaffolds in osteochondral repair. , Orthop. Clin. North Am 43, 255-261.
- 35.Vanderleyden E, Mullens S, Luyten J, Dubruel P. (2012) Implantable (bio)polymer coated titanium scaffolds: a review. , Curr. Pharm. Des 18, 2576-2590.
- 37.Rezwan K, Q Z Chen, J, A R Boccaccini. (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 27, 3413-3431.
- 38.D, N D Katsala, P G Koutsoukos, Y F Missirlis. (2001) Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 22, 87-96.
- 39.Fini M, Giardino R, Borsari V, Torricelli P, Rimondini L et al. (2003) In vitro behaviour of osteoblasts cultured on orthopaedic biomaterials with different surface roughness, uncoated and fluorohydroxyapatite-coated, relative to the in vivo osteointegration rate. , Int. J. Artif. Organs 26, 520-528.
- 40.Sato M, T J Webster. (2006) Designing orthopedic implant surfaces: harmonization of nanotopographical and chemical aspects. Nanomedicine. 1, 351-354.
- 41.Li X, Blitterswijk C A van, Feng Q, Cui F, Watari F. (2008) The effect of calcium phosphate microstructure on bone-related cells in vitro. Biomaterials. 29, 3306-3316.
- 42.Kumar G, M S Waters, T M Farooque, M F Young, C G Simon. (2012) Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials. 33, 4022-4030.
- 43.M G Holthaus, Treccani L, Rezwan K.Osteoblast viability on hydroxyapatite with well-adjusted submicron and micron surface roughness as monitored by the proliferation reagent WST2-1. , J. Biomater. Appl 2013, 791-800.
- 44.Karageorgiou V, Kaplan D. (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 26, 5474-5491.
- 45.Huebsch N, D J Mooney. (2009) Inspiration and application in the evolution of biomaterials. , Nature 462-426.
- 46.Zhou Y, Chen F, S T Ho, M A Woodruff, T M Lim et al. (2007) Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials. 28, 814-824.
- 47.Vitale-Brovarone C, Baino F, Verné E. (2009) High strength bioactive glass-ceramic scaffolds for bone regeneration. , J. Mater. Sci. Mater. Med 20, 643-653.
- 48.Ebaretonbofa E, J R Evans.High porosity hydroxyapatite foam scaffolds for bone substitute. , J. Porous Mater 2002, 257-263.
- 49.Specchia N, Pagnotta A, Cappella M, Tampieri A, Greco F. (2002) Effect of hydroxyapatite porosity on growth and differentiation of human osteoblast-like cells. , J. Mater. Sci 37, 577-584.
- 50.K A Hing. (2005) Bioceramic bone graft substitutes: influence of porosity and chemistry. , Int. J. Appl. Ceram. Technol 2, 184-199.
- 51.Malmstrom J, Adolfsson E, Arvidsson A, Thomsen P.Bone response inside free-form fabricated macroporous hydroxyapatite scaffolds with and without an open microporosity. , Clin. Implant Dent. Rel. Res 2007, 79-88.
- 52.Peng Q, Jiang F, Huang P, Zhou S, Weng J et al. (2010) A novel porous bioceramics scaffold by accumulating hydroxyapatite spherules for large bone tissue engineering in vivo. I. Preparation and characterization of scaffold. , J. Biomed. Mater. Res. A 93-920.
- 53.K S Lew, Othman R, Ishikawa K, F Y Yeoh. (2012) Macroporous bioceramics: a remarkable material for bone regeneration. , J. Biomater. Appl 27, 345-358.
- 54.L M Ren, Todo M, Arahira T, Yoshikawa H, Myoui A.A comparative biomechanical study of bone ingrowth in two porous hydroxyapatite bioceramics. , Appl. Surf. Sci 2012, 81-88.
- 55.Guda T, J A Walker, Singleton B, Hernandez J, D S Oh et al.Hydroxyapatite scaffold pore architecture effects in large bone defects in vivo. , J. Biomater. Appl 2014, 1016-1027.
- 56.Shao R, Quan R, Zhang L, Wei X, Yang D et al.Porous hydroxyapatite bioceramics in bone tissue engineering: current uses and perspectives. , J. Ceram. Soc. Jpn 2015, 17-20.
- 59.Artzi Z, Weinreb M, Givol N, Rohrer Nemcovsky, C E Prasad et al. (2004) Biomaterial resorbability and healing site morphology of inorganic bovine bone and beta tricalcium phosphate in the canine: a 24-month longitudinal histologic study and morphometric analysis. , Int. J. Oral Max. Impl 19, 357-368.
- 60.Burg K J L, Porter S, J F Kellam. (2000) Biomaterial developments for bone tissue engineering. Biomaterials. 21, 2347-2359.
- 61.S V Dorozhkin.Self-setting calcium orthophosphate formulations. , J. Funct. Biomater 2013, 209-311.
- 62.T, R W Smith. (2009) Employing the Taguchi method in optimizing the scaffold production process for artificial bone grafts. , J. Mater. Process. Technol 209-1521.
- 64.Daculsi G, Miramond T, Borget P, Baroth S. (2013) Smart calcium phosphate bioceramic scaffold for bone tissue engineering. Key Eng. , Mater 529-530.
- 65.Vallet-Regí M. (2008) Bioceramics: where do we come from and which are the future expectations. Key Eng. , Mater 377, 1-18.
- 66.Sakamoto M, Matsumoto T. (2012) Development and evaluation of superporous ceramics bone tissue scaffold materials with triple pore structure a) hydroxyapatite, b) beta-tricalcium phosphate. In: Bone. , Rijeka, Croatia 301-320.
- 67.Bohner M, Loosli Y, Baroud G, D Commentary Lacroix. (2011) Deciphering the link between architecture and biological response of a bone graft substitute. Acta Biomater. 7, 478-484.
- 68.Balazsi C, Weber F, Kover Z, Horvath E, Nemeth C. (2007) Preparation of calcium-phosphate bioceramics from natural resources. , J. Eur. Ceram. Soc 27, 1601-1606.
- 69.Gergely G, Wéber F, Lukács I, Illés L, A L Tóth et al.Nano-hydroxyapatite preparation from biogenic raw materials. , Cent. Eur. J. Chem 2010, 375-381.
- 70.Mondal S, Mahata S, Kundu S, Mondal B.Processing of natural resourced hydroxyapatite ceramics from fish scale. , Adv. Appl. Ceram 2010, 234-239.
- 71.K T Lim, J D Suh, Kim J, P H Choung, J H Chung.Calcium phosphate bioceramics fabricated from extracted human teeth for tooth tissue engineering. , J. Biomed. Mater. Res. B Appl. Biomater 2011, 399-411.
- 72.D S Seo, K H Hwang, S Y Yoon, J K Lee.Fabrication of hydroxyapatite bioceramics from the recycling of pig bone. , J. Ceram. Proc. Res 2012, 586-589.
- 73.W F Ho, H C, S K Hsu, C W Hung, S C Wu.Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. , Ceram. Int 2013, 6467-6473.
- 74.Salma-Ancane K, Stipniece L, Irbe Z. (2016) Effect of biogenic and synthetic starting materials on the structure of hydroxyapatite bioceramics. , Ceram. Int 42, 9504-9510.
- 75.A C Taş, Korkusuz F, Timuçin M, Akkaş N. (1997) An investigation of the chemical synthesis and high-temperature sintering behaviour of calcium hydroxyapatite (HA) and tricalcium phosphate (TCP). 8, 91-96.
- 76.Layrolle P, Ito A, Tateishi T. (1998) Sol-gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. , J. Am. Ceram. Soc 81, 1421-1428.
- 77.N O Engin, A C Tas. (1999) Manufacture of macroporous calcium hydroxyapatite bioceramics. , J. Eur. Ceram. Soc 19, 2569-2572.
- 78.E S Ahn, N J Gleason, Nakahira A, J Y Ying. (2001) Nanostructure processing of hydroxyapatite-based bioceramics. Nano Lett. 1, 149-153.
- 79.K A, S W Kim, Dharmaraj N, K W, H Y Kim. (2007) Novel mechanism to improve toughness of the hydroxyapatite bioceramics using high-frequency induction heat sintering. , J. Mater. Process. Technol 187-188.
- 80.Laasri S, Taha M, Laghzizil A, E K Hlil, Chevalier J.The affect of densification and dehydroxylation on the mechanical properties of stoichiometric hydroxyapatite bioceramics. , Mater. Res. Bull 2010, 1433-1437.
- 81.Kitamura M, Ohtsuki C, Ogata S, Kamitakahara M, Tanihara M. (2004) Microstructure and bioresorbable properties of α-TCP ceramic porous body fabricated by direct casting method. , Mater. Trans 45, 983-988.
- 82.Kawagoe D, Ioku K, Fujimori H, Goto S. (2004) Transparent β-tricalcium phosphate ceramics prepared by spark plasma sintering. , J. Ceram. Soc. Jpn 112-462.
- 83.C X Wang, Zhou X, Wang M. (2004) Influence of sintering temperatures on hardness and Young’s modulus of tricalcium phosphate bioceramic by nanoindentation technique. , Mater. Character 52, 301-307.
- 84.Ioku K, Kawachi G, Nakahara K, E H Ishida, Minagi H et al. (2006) Porous granules of β-tricalcium phosphate composed of rod-shaped particles. , Key Eng. Mater 309-311.
- 85.Kamitakahara M, Ohtsuki C, Miyazaki T. (2008) Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. , J. Biomater. Appl 23, 197-212.
- 86.Vorndran E, Klarner M, Klammert U, L M Grover, Patel S et al. (2008) 3D powder printing of β-tricalcium phosphate ceramics using different strategies. , Adv. Eng. Mater 10, 67-71.
- 87.Descamps M, Duhoo T, Monchau F, Lu J, Hardouin P et al. (2008) Manufacture of macroporous β-tricalcium phosphate bioceramics. , J. Eur. Ceram. Soc 28, 149-157.
- 88.Liu Y, J H Kim, Young D, Kim S, S K Nishimoto et al. (2010) Novel template-casting technique for fabricating β-tricalcium phosphate scaffolds with high interconnectivity and mechanical strength and in vitro cell responses. , J. Biomed. Mater. Res. A 92-997.
- 89.R G Carrodeguas, S de Aza. (2011) α-tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 7, 3536-3546.
- 90.Zhang Y, Kong D, Feng X.Fabrication and properties of porous β-tricalcium phosphate ceramics prepared using a double slip-casting method using slips with different viscosities. , Ceram. Int 2012, 2991-2996.
- 91.I Y Kim, Wen J, Ohtsuki C. (2015) Fabrication of α-tricalcium phosphate ceramics through two-step sintering. Key Eng. , Mater 631-78.
- 92.S V Dorozhkin. (2016) Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. , Ceram. Int 42, 6529-6554.
- 93.LeGeros R Z Lin, Rohanizadeh S, Mijares R, LeGeros D, P J. (2003) Biphasic calcium phosphate bioceramics: preparation, properties and applications. 14, 201-209.
- 94.Daculsi G, Laboux O, Malard O, Weiss P. (2003) Current state of the art of biphasic calcium phosphate bioceramics. , J. Mater. Sci. Mater. Med 14, 195-200.
- 95.E I Dorozhkina, S V Dorozhkin. (2002) Mechanism of the solid-state transformation of a calcium-deficient hydroxyapatite (CDHA) into biphasic calcium phosphate (BCP) at elevated temperatures. , Chem. Mater 14, 4267-4272.
- 96.Daculsi G. (2006) Biphasic calcium phosphate granules concept for injectable and mouldable bone substitute. , Adv. Sci. Technol 49, 9-13.
- 97.Lecomte A, Gautier H, J M Bouler, Gouyette A, Pegon Y et al. (2008) Biphasic calcium phosphate: a comparative study of interconnected porosity in two ceramics. , J. Biomed. Mater. Res. B Appl. Biomater 84, 1-6.
- 98.Daculsi G, Baroth S, R Z LeGeros. (2010) 20 years of biphasic calcium phosphate bioceramics development and applications. Ceram. Eng. Sci. Proc 30, 45-58.
- 99.Lukić M, Stojanović Z, S D Škapin, Maček-Kržmanc M, Mitrić M et al. () Dense fine-grained biphasic calcium phosphate (BCP) bioceramics designed by two-step sintering. , J. Eur. Ceram. Soc 2011.
- 100.Descamps M, Boilet L, Moreau G, Tricoteaux A, Lu J et al.Processing and properties of biphasic calcium phosphates bioceramics obtained by pressureless sintering and hot isostatic pressing. , J. Eur. Ceram. Soc 2013, 1263-1270.
- 101.Chen Y, Wang J, X D Zhu, Z R Tang, Yang X et al. (2015) Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: in vitro and in vivo evidence. Acta Biomater. 11, 435-448.
- 102.Li Y, Kong F, Weng W. (2009) Preparation and characterization of novel biphasic calcium phosphate powders (α-TCP/HA) derived from carbonated amorphous calcium phosphates. , J. Biomed. Mater. Res. B Appl. Biomater 89-508.
- 103.Sureshbabu S, Komath M, H K Varma.In situ formation of hydroxyapatite –alpha tricalcium phosphate biphasic ceramics with higher strength and bioactivity. , J. Am. Ceram. Soc 2012, 915-924.
- 104.Radovanović Ž, Jokić B, Veljović D, Dimitrijević S, Kojić V et al.Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. , Appl. Surf. Sci 2014, 513-519.
- 105.Oishi M, Ohtsuki C, Kitamura M, Kamitakahara M, Ogata S et al. (2004) Fabrication and chemical durability of porous bodies consisting of biphasic tricalcium phosphates. Phosphorus Res. , Bull 17, 95-100.
- 106.Kamitakahara M, Ohtsuki C, Oishi M, Ogata S, Miyazaki T et al. (2005) Preparation of porous biphasic tricalcium phosphate and its in vivo behavior. Key Eng. , Mater 284-286.
- 107.Wang R, Weng W, Deng X, Cheng K, Liu X et al. (2006) Dissolution behavior of submicron biphasic tricalcium phosphate powders. Key Eng. , Mater 309-311.
- 108.Li Y, Weng W, K C Tam. (2007) Novel highly biodegradable biphasic tricalcium phosphates composed of α-tricalcium phosphate and β-tricalcium phosphate. Acta Biomater. 3, 251-254.
- 109.Zou C, Cheng K, Weng W, Song C, Du P et al.Characterization and dissolution–reprecipitation behavior of biphasic tricalcium phosphate powders. , J. Alloys Compd 2011, 6852-6858.
- 110.Xie L, Yu H, Deng Y, Yang W, Liao L et al. (2016) Preparation and in vitro degradation study of the porous dual alpha/beta-tricalcium phosphate bioceramics. , Mater. Res. Inn 20, 530-537.
- 111.Albuquerque J S V, Nogueira R E F Q, Silva da, Lima T D P, O da Silva D et al. (2004) Porous triphasic calcium phosphate bioceramics. Key Eng. , Mater 254-256.
- 112.Mendonça F, Lourom L H L, Campos J B de, Silva da, M H P. (2008) Porous biphasic and triphasic bioceramics scaffolds produced by gelcasting. Key Eng. , Mater 361-363.
- 113.Vani R, E K Girija, Elayaraja K, P S, Kesavamoorthy R et al. (2009) Hydrothermal synthesis of porous triphasic hydroxyapatite/(α and β) tricalcium phosphate. , J. Mater. Sci. Mater. Med 20, 43-48.
- 114.M K Ahn, Y W Moon, Y H Koh, H E Kim.Production of highly porous triphasic calcium phosphate scaffolds with excellent in vitro bioactivity using vacuum-assisted foaming of ceramic suspension (VFC) technique. , Ceram. Int 2013, 5879-5885.
- 115.Tamimi F, Sheikh Z, Barralet J. (2012) Dicalcium phosphate cements: brushite and monetite. Acta Biomater. 8, 474-487.
- 116.Drouet C, Largeot C, Raimbeaux G, Estournès C, Dechambre G et al. (2006) Bioceramics: spark plasma sintering (SPS) of calcium phosphates. , Adv. Sci. Technol 49, 45-50.
- 117.Ishihara S, Matsumoto T, Onoki T, Sohmura T, Nakahira A. (2009) New concept bioceramics composed of octacalcium phosphate (OCP) and dicarboxylic acid-intercalated OCP via hydrothermal hot-pressing. , Mater. Sci. Eng. C 29, 1885-1888.
- 118.S M Barinov, V S Komlev.Osteoinductive ceramic materials for bone tissue restoration: octacalcium phosphate (review). , Inorg. Mater. Appl. Res 2010, 175-181.
- 119.Moseke C, Gbureck U. (2010) Tetracalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 6, 3815-3823.
- 120.Morimoto S, Anada T, Honda Y, Suzuki O.Comparative study on in vitro biocompatibility of synthetic octacalcium phosphate and calcium phosphate ceramics used clinically. , Biomed. Mater 2012, 045020.
- 121.Tamimi F, D L Nihouannen, Eimar H, Sheikh Z, Komarova S et al. (2012) The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: brushite vs. monetite. Acta Biomater. 8, 3161-3169.
- 122.Suzuki O. (2013) Octacalcium phosphate (OCP)-based bone substitute materials. , Jpn. Dent. Sci. Rev 49, 58-71.
- 123.Suzuki O, Anada T. (2014) Octacalcium phosphate: a potential scaffold material for controlling activity of bone-related cells in vitro. , Mater. Sci. Forum 783-786.
- 124.V S Komlev, S M Barinov, I, R V Deev, Eremin.Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behavior. , I.I., Fedotov, A.Y., Gurin, A.N., Khromova, N.V., Kopnin, P.B., Kuvshinova, E.A., Mamonov, V.E., Rybko, V.A., Sergeeva, N.S., Teterina, A.Y., Zorin, V.L, ACS Appl. Mater. Interf 2014, 16610-16620.
- 125.R Z LeGeros. (1991) Calcium phosphates in oral biology and medicine. Monographs in oral science. , Karger, Basel, Switzerland 15, 201.
- 126.Narasaraju T S B, D E Phebe. (1996) Some physico-chemical aspects of hydroxylapatite. , J. Mater. Sci 31, 1-21.
- 127.J C Elliott. (1994) Structure and chemistry of the apatites and other calcium orthophosphates. Studies in inorganic chemistry , Amsterdam, Netherlands 18, 389.
- 130.Silva da, R V Bertran, C A Kawachi, E Y Camilli, A J. (2007) Repair of cranial bone defects with calcium phosphate ceramic implant or autogenous bone. 18, 281-286.
- 131.Okanoue Y, Ikeuchi M, Takemasa R, Tani T, Matsumoto T et al.Comparison of in vivo bioactivity and compressive strength of a novel superporous hydroxyapatite with beta-tricalcium phosphates. , Arch. Orthop. Trauma Surg 2012, 1603-1610.
- 132.Draenert M, Draenert A, Draenert K. (2013) Osseointegration of hydroxyapatite and remodeling-resorption of tricalciumphosphate ceramics. , Microsc. Res. Tech 76, 370-380.
- 133.Okuda T, Ioku K, Yonezawa I, Minagi H, Gonda Y et al. (2008) The slow resorption with replacement by bone of a hydrothermally synthesized pure calcium-deficient hydroxyapatite. Biomaterials. 29, 2719-2728.
- 134.Daculsi G, J M Bouler, R Z LeGeros. (1997) Adaptive crystal formation in normal and pathological calcifications in synthetic calcium phosphate and related biomaterials. , Int. Rev. Cytol 172-129.
- 135.X D Zhu, H J Zhang, H S Fan, Li W, X D Zhang. (2010) Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 6, 1536-1541.
- 136.Bohner M. (2000) Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 31, 37-47.
- 138.Popişter F, Popescu D, Hurgoiu D. (2012) A new method for using reverse engineering in case of ceramic tiles. , Qual. Access Success 13, 409-412.
- 139.Yang S, K F Leong, Du Z, C K. (2002) The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 8, 1-11.
- 140.W Y Yeong, C K, K F Leong, Chandrasekaran M. (2004) Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 22, 643-652.
- 141.Ortona A, D’Angelo C, Gianella S, Gaia D. (2012) Cellular ceramics produced by rapid prototyping and replication. 80, 95-98.
- 142.Eufinger H, Wehniöller M, Machtens E, Heuser L, Harders A et al. (1995) Reconstruction of craniofacial bone defects with individual alloplastic implants based on CAD/CAM-manipulated CT-data. , J. Cranio Maxillofac. Surg 23, 175-181.
- 143.Klein M, Glatzer C. (2006) Individual CAD/CAM fabricated glass-bioceramic implants in reconstructive surgery of the bony orbital floor. Plastic Reconstruct. Surg. 117-565.
- 144.Yin L, X F Song, Y L Song, Huang T, Li J. (2006) An overview of in vitro abrasive finishing & CAD/CAM of bioceramics in restorative dentistry. , Int. J. Machine Tools Manufact 46, 1013-1026.
- 145.Li J, Hsu Y, Luo E, Khadka A, Hu J. (2011) Computer-aided design and manufacturing and rapid prototyped nanoscale hydroxyapatite/polyamide (n-HA/PA) construction for condylar defect caused by mandibular angle ostectomy. Aesthetic Plast. Surg. 35, 636-640.
- 146.Ciocca L, Donati D, Fantini M, Landi E, Piattelli A et al.CAD-CAM-generated hydroxyapatite scaffold to replace the mandibular condyle in sheep: preliminary results. , J. Biomater. Appl 2013, 207-218.
- 147.M A Yardimci, S I Guceri, S C Danforth. (1996) Process modeling for fused deposition of ceramics. , Ceram. Eng. Sci. Proc 17, 78-82.
- 148.Bellini A, Shor L, S I Guceri. (2005) New developments in fused deposition modeling of ceramics. Rapid Prototyping. 11, 214-220.
- 149.K H Tan, C K, K F Leong, C M, Cheang P et al. (2003) Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 24, 3115-3123.
- 150.F E Wiria, K F Leong, C K, Liu Y. (2007) Poly-ε-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 3, 1-12.
- 151.Xiao K, K W Dalgarno, D J Wood, R D Goodridge, Ohtsuki C. (2008) Indirect selective laser sintering of apatite-wollostonite glass-ceramic. , Proc. Inst. Mech. Eng. H 222-1107.
- 152.W Y Zhou, S H Lee, Wang M, W L Cheung, W Y Ip. (2008) Selective laser sintering of porous tissue engineering scaffolds from poly(L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. , J. Mater. Sci. Mater. Med 19, 2535-2540.
- 153.C J Shuai, P J Li, Feng P, H B Lu, S P Peng et al. (2013) Analysis of transient temperature distribution during the selective laser sintering of β-tricalcium phosphate. Laser Eng. 26, 71-80.
- 154.Shuai C, Zhuang J, Hu H, Peng S, Liu D et al.In vitro bioactivity and degradability of β-tricalcium phosphate porous scaffold fabricated via selective laser sintering. , Biotechnol. Appl. Biochem 2013, 266-273.
- 155.Shuai C, Zhuang J, Peng S, Wen X. (2014) Inhibition of phase transformation from β- to α-tricalcium phosphate with addition of poly (L-lactic acid) in selective laser sintering. Rapid Prototyping. 20, 369-376.
- 156.Lusquiños F, Pou J, Boutinguiza M, Quintero F, Soto R et al. (2005) Main characteristics of calcium phosphate coatings obtained by laser cladding. , Appl. Surf. Sci 247-486.
- 157.D G Wang, C Z, Ma J, Zhang G. (2008) In situ synthesis of hydroxyapatite coating by laser cladding. Colloid Surf. B. 66, 155-162.
- 158.Comesaña R, Lusquiños F, Val del, Malot J, López-Álvarez T et al.Calcium phosphate grafts produced by rapid prototyping based on laser cladding. , J. Eur. Ceram. Soc 2011, 29-41.
- 159.Lv X, Lin X, Hu J, Gao B, Huang W. (2012) Phase evolution in calcium phosphate coatings obtained by in situ laser cladding. , Mater. Sci. Eng. C 32, 872-877.
- 160.Leukers B, Gülkan H, S H Irsen, Milz S, Tille C et al. (2005) Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. , J. Mater. Sci. Mater. Med 16, 1121-1124.
- 161.Gbureck U, Hölzel T, Klammert U, Würzler K, F A Müller et al. (2007) Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. , Adv. Funct. Mater 17, 3940-3945.
- 162.Gbureck U, Hölzel T, C J Doillon, F A Müller, J E Barralet. (2007) Direct printing of bioceramic implants with spatially localized angiogenic factors. , Adv. Mater 19, 795-800.
- 163.Khalyfa A, Vogt S, Weisser J, Grimm G, Rechtenbach A et al. (2007) Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. , J. Mater. Sci. Mater. Med 18, 909-916.
- 164.Habibovic P, Gbureck U, C J Doillon, D C Bassett, Blitterswijk C A van et al. (2008) Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 29, 944-953.
- 165.F C, Beckmann F, Huser M, S H Irsen, Leukers B et al. (2008) The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials. 29, 3799-3806.
- 166.Seitz H, Deisinger U, Leukers B, Detsch R, Ziegler G. (2009) Different calcium phosphate granules for 3-D printing of bone tissue engineering scaffolds. , Adv. Eng. Mater 11, 41-46.
- 167.Suwanprateeb J, Sanngam R, Panyathanmaporn T. (2010) Influence of raw powder preparation routes on properties of hydroxyapatite fabricated by 3D printing technique. , Mater. Sci. Eng. C 30, 610-617.
- 168.Butscher A, Bohner M, Roth C, Ernstberger A, Heuberger R et al. (2012) Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 8, 373-385.
- 169.Butscher A, Bohner M, Doebelin N, Galea L, Loeffel O et al. (2013) Moisture based three-dimensional printing of calcium phosphate structures for scaffold engineering. Acta Biomater. 9, 5369-5378.
- 170.Butscher A, Bohner M, Doebelin N, Hofmann S, Müller R. (2013) New depowdering-friendly designs for three-dimensional printing of calcium phosphate bone substitutes. Acta Biomater. 9, 9149-9158.
- 171.Tarafder S, N M Davies, Bandyopadhyay A, Bose S.3D printed tricalcium phosphate bone tissue engineering scaffolds: effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. , Biomater. Sci 2013, 1250-1259.
- 172.Maazouz Y, E B Montufar, Guillem-Marti J, Fleps I, Öhman C et al. (2014) Robocasting of biomimetic hydroxyapatite scaffolds using self-setting inks. , J. Mater. Chem. B 2, 5378-5386.
- 173.A R, Luo Y, Schumacher M, Nies B, Lode A et al. (2015) 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater. 27, 264-274.
- 174.Trombetta R, J A Inzana, E M Schwarz, S L Kates, H A Awad.3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. , Ann. Biomed. Eng 2017, 23-44.
- 175.N L Porter, R M Pilliar, Grynpas. (2001) Fabrication of porous calcium polyphosphate implants by solid freeform fabrication: a study of processing parameters and in vitro degradation characteristics. , J. Biomed. Mater. Res 56, 504-515.
- 176.K F Leong, C M, C K. (2003) Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 24, 2363-2378.
- 177.J W Calvert, K A Brenner, M P, Kumta P, L E Weiss. (2004) Cellular fusion of hydroxyapatite layers: solid freeform fabrication of synthetic bone grafts. , Plast, Riv. Ital. Chir 36, 145-150.
- 178.Jongpaiboonkit L, C Y Lin, P H Krebsbach, S J Hollister, J W Halloran. (2006) Mechanical behavior of complex 3D calcium phosphate cement scaffolds fabricated by indirect solid freeform fabrication in. 309-311.
- 179.D R. (2007) Robotic deposition of model hydroxyapatite scaffolds with multiple architectures and multiscale porosity for bone tissue engineering. , J. Biomed. Mater. Res. A 82-383.
- 180.Shanjani Y, Croos J N A de, R M Pilliar, R A Kandel, Toyserkani E.Solid freeform fabrication and characterization of porous calcium polyphosphate structures for tissue engineering purposes. , J. Biomed. Mater. Res. B Appl. Biomater 2010, 510-519.
- 181.Kim J, Lim D, Y H Kim, Y H Koh, M H Lee et al.A comparative study of the physical and mechanical properties of porous hydroxyapatite scaffolds fabricated by solid freeform fabrication and polymer replication method. , Int. J. Precision Eng. Manuf 2011, 695-701.
- 182.Shanjani Y, Hu Y, Toyserkani E, Grynpas M, R A Kandel et al.Solid freeform fabrication of porous calcium polyphosphate structures for bone substitute applications: in vivo studies. , J. Biomed. Mater. Res. B Appl. Biomater 2013, 972-980.
- 183.B J Kwon, Kim J, Y H Kim, M H Lee, H S Baek et al. (2013) Biological advantages of porous hydroxyapatite scaffold made by solid freeform fabrication for bone tissue regeneration. , Artif. Organs 37, 663-670.
- 184.Li X, Li D, Lu B, Wang C. (2008) Fabrication of bioceramic scaffolds with pre-designed internal architecture by gel casting and indirect stereolithography techniques. 15, 667-671.
- 185.Maeda C, Tasaki S, Kirihara S.Accurate fabrication of hydroxyapatite bone models with porous scaffold structures by using stereolithography. , IOP Conf. Ser. Mater. Sci. Eng 2011, 072017.
- 186.Bian W, Li D, Lian Q, Li X, Zhang W et al. (2012) Fabrication of a bio-inspired beta-tricalcium phosphate/collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyping. 18, 68-80.
- 187.Ronca A, Ambrosio L, D W Grijpma. (2013) Preparation of designed poly(D,L-lactide)/nanosized hydroxyapatite composite structures by stereolithography. Acta Biomater. 9, 5989-5996.
- 188.Saber-Samandari S, K A Gross. (2010) The use of thermal printing to control the properties of calcium phosphate deposits. Biomaterials. 31, 6386-6393.
- 189.Meira C R de, D T Gomes, Braga F J C, de Moraes Purquerio, Fortulan B et al. (2015) Direct manufacture of hydroxyapatite scaffolds using blue laser. , Mater. Sci. Forum 805-128.
- 190.R J Narayan, Jin C, Doraiswamy A, I N Mihailescu, Jelinek M et al.Laser processing of advanced bioceramics. , Adv. Eng. Mater 2005, 1083-1098.
- 191.Nather A. (2005) Bone grafts and bone substitutes: basic science and clinical applications. World Scientific. , Singapore 592.
- 192.Bártolo P, Bidanda B. (2008) Bio-materials and prototyping applications in medicine. , New York, USA 216.
- 195.Raksujarit A, Pengpat K, Rujijanagul G, Tunkasiri T. (2010) Processing and properties of nanoporous hydroxyapatite ceramics. 31, 1658-1660.
- 196.Park J. (2008) Bioceramics: properties, characterizations, and applications. , New York, USA 364.
- 197.L M Rodriguez-, Vallet-Regi M, Ferreira J M F. (2001) Fabrication of hydroxyapatite bodies by uniaxial pressing from a precipitated powder. Biomaterials. 22, 583-588.
- 198.Miranda P, Pajares A, Saiz E, A P Tomsia, Guiberteau F. (2007) Mechanical behaviour under uniaxial compression of robocast calcium phosphate scaffolds. Eur. Cells Mater 14, 79.
- 199.M H Nazarpak, Solati-Hashjin M, Moztarzadeh F. (2009) Preparation of hydroxyapatite ceramics for biomedical applications. , J. Ceram. Process. Res 10, 54-57.
- 200.Uematsu K, Takagi M, Honda T, Uchida N, Saito K. (1989) Transparent hydroxyapatite prepared by hot isostatic pressing of filter cake. , J. Am. Ceram. Soc 72, 1476-1478.
- 201.Itoh H, Wakisaka Y, Ohnuma Y, Kuboki Y. (1994) A new porous hydroxyapatite ceramic prepared by cold isostatic pressing and sintering synthesized flaky powder. , Dent. Mater 13, 25-35.
- 202.Takikawa K, Akao M. (1996) Fabrication of transparent hydroxyapatite and application to bone marrow derived cell/hydroxyapatite interaction observation in-vivo. , J. Mater. Sci. Mater. Med 7, 439-445.
- 203.Gautier H, Merle C, J L Auget, Daculsi G. (2000) Isostatic compression, a new process for incorporating vancomycin into biphasic calcium phosphate: comparison with a classical method. Biomaterials. 21, 243-249.
- 204.Tadic D, Epple M. (2003) Mechanically stable implants of synthetic bone mineral by cold isostatic pressing. Biomaterials. 24, 4565-4571.
- 205.Onoki T, Hashida T. (2006) New method for hydroxyapatite coating of titanium by the hydrothermal hot isostatic pressing technique. , Surf. Coat. Technol 200, 6801-6807.
- 206.Ergun C. (2011) Enhanced phase stability in hydroxylapatite/zirconia composites with hot isostatic pressing. , Ceram. Int 37, 935-942.
- 207.Ehsani N, A J Ruys, C.Hot isostatic pressing (HIPing) of fecralloy-reinforced hydroxyapatite. , J. Biomimet. Biomater. Tissue Eng 2013, 87-102.
- 208.S H Irsen, Leukers B, Höckling Chr, Tille C, Seitz H. (2006) Bioceramic granulates for use in 3D printing: process engineering aspects. , Materwiss. Werksttech 37, 533-537.
- 209.Y H Hsu, I G Turner, A W Miles. (2007) Fabrication and mechanical testing of porous calcium phosphate bioceramic granules. , J. Mater. Sci. Mater. Med 18, 1931-1937.
- 210.Z, Glushko V, Dedukh N, Malyshkina S, Ashukina N. (2008) Porous calcium phosphate ceramic granules and their behaviour in differently loaded areas of skeleton. , J. Mater. Sci. Mater. Med 19, 2197-2205.
- 211.Viana M, Désiré A, Chevalier E, Champion E, Chotard R et al. (2009) Interest of high shear wet granulation to produce drug loaded porous calcium phosphate pellets for bone filling. Key Eng. , Mater 396-398.
- 212.Chevalier E, Viana M, Cazalbou S, Chulia D. (2009) Comparison of low-shear and high-shear granulation processes: effect on implantable calcium phosphate granule properties. , Drug Dev. Ind. Pharm 35, 1255-1263.
- 213.Lakevics V, Locs J, Loca D, Stepanova V, Berzina-Cimdina L et al. (2011) Bioceramic hydroxyapatite granules for purification of biotechnological products. , Adv. Mater. Res 284-286.
- 214.Camargo N H A, P F Franczak, Gemelli E, Costa da, Moraes B D de et al.Characterization of three calcium phosphate microporous granulated bioceramics. , Adv. Mater. Res 2014, 687-694.
- 215.Reikerås O, C B Johansson, Sundfeldt M. (2006) Bone ingrowths to press-fit and loose-fit implants: comparisons between titanium and hydroxyapatite. , J. Long-Term Eff. Med. Implant 16, 157-164.
- 216.R, T S Kannan. (2001) Dispersion and slip casting of hydroxyapatite. , J. Am. Ceram. Soc 84, 1710-1716.
- 217.Sakka Y, Takahashi K, Matsuda N, T S Suzuki. (2007) Effect of milling treatment on texture development of hydroxyapatite ceramics by slip casting in high magnetic field. , Mater. Trans 48, 2861-2866.
- 218.Zhang Y, Yokogawa Y, Feng X, Tao Y, Li Y.Preparation and properties of bimodal porous apatite ceramics through slip casting using different hydroxyapatite powders. , Ceram. Int 2010, 107-113.
- 219.Zhang Y, Kong D, Yokogawa Y, Feng X, Tao Y et al.Fabrication of porous hydroxyapatite ceramic scaffolds with high flexural strength through the double slip-casting method using fine powders. , J. Am. Ceram. Soc 2012, 147-152.
- 220.Hagio T, Yamauchi K, Kohama T, Matsuzaki T, Iwai K. (2013) Beta tricalcium phosphate ceramics with controlled crystal orientation fabricated by application of external magnetic field during the slip casting process. , Mater. Sci. Eng. C 33, 2967-2970.
- 221.Marçal R L S B, Rocha da, N da Silva D, M H P.Slip casting used as a forming technique for hydroxyapatite processing. , Key Eng. Mater 2017, 219-222.
- 222.Sepulveda P, F S Ortega, Innocentini M D M, V C Pandolfelli. (2000) Properties of highly porous hydroxyapatite obtained by the gel casting of foams. , J. Am. Ceram. Soc 83, 3021-3024.
- 223.Padilla S, Vallet-Regi M, M P Ginebra, F J Gil. (2005) Processing and mechanical properties of hydroxyapatite pieces obtained by the gel-casting method. , J. Eur. Ceram. Soc 25, 375-383.
- 224.Woesz A, Rumpler M, Stampfl J, Varga F, Fratzl-Zelman N et al. (2005) Towards bone replacement materials from calcium phosphates via rapid prototyping and ceramic gel casting. , Mater. Sci. Eng. C 25, 181-186.
- 225.Sanchez-Salcedo S, Werner J, Vallet-Regi M. (2008) Hierarchical pore structure of calcium phosphate scaffolds by a combination of gel-casting and multiple tape-casting methods. Acta Biomater. 4, 913-922.
- 226.Chen B, Zhang T, Zhang J, Lin Q, Jiang D. (2008) Microstructure and mechanical properties of hydroxyapatite obtained by gel-casting process. , Ceram. Int 34, 359-364.
- 227.Marcassoli P, Cabrini M, Tirillò J, Bartuli C, Palmero P et al. (2010) Mechanical characterization of hydroxiapatite micro/macro-porous ceramics obtained by means of innovative gel-casting process. Key Eng. , Mater 417-418.
- 228.T W Kim, S C Ryu, B K Kim, S Y Yoon, H C Park.Porous hydroxyapatite scaffolds containing calcium phosphate glass-ceramics processed using a freeze/gel-casting technique. , Metal Mater. Int 2014, 135-140.
- 229.Asif A, Nazir R, Riaz T, Ashraf N, Zahid S et al. (2014) Influence of processing parameters and solid concentration on microstructural properties of gel-casted porous. 21, 31-37.
- 230.S R Dash, Sarkar R, Bhattacharyya S. (2015) Gel casting of hydroxyapatite with naphthalene as pore former. , Ceram. Int 41, 3775-3790.
- 231.A S Fomin, S M Barinov, V M Ievlev, V, B P Mikhailov et al. (2008) Nanocrystalline hydroxyapatite ceramics produced by low-temperature sintering after high-pressure treatment. , Dokl. Chem 418-22.
- 232.Zhang J, H M Yin, B S Hsiao, G J Zhong, Z M Li. (2014) Biodegradable poly(lactic acid)/hydroxyl apatite 3D porous scaffolds using high-pressure molding and salt. 49, 1648-1658.
- 233.Kankawa Y, Kaneko Y, Saitou K. (1991) Injection molding of highly-purified hydroxylapatite and TCP utilizing solid phase reaction method. , J. Ceram. Soc. Jpn 99, 438-442.
- 234.Cihlář J, Trunec M. (1996) Injection moulded hydroxyapatite ceramics. Biomaterials. 17, 1905-1911.
- 235.Jewad R, Bentham C, Hancock B, Bonfield W, S M Best. (2008) Dispersant selection for aqueous medium pressure injection moulding of anhydrous dicalcium phosphate. , J. Eur. Ceram. Soc 28, 547-553.
- 236.S H Kwon, Y K Jun, S H Hong, I S Lee, H E Kim et al. (2002) Calcium phosphate bioceramics with various porosities and dissolution rates. , J. Am. Ceram. Soc 85, 3129-3131.
- 237.Fooki A C B M, A H, T B Fideles, R C Costa, Fook M V L. (2009) Porous hydroxyapatite scaffolds by polymer sponge method. Key Eng. , Mater 396-398.
- 238.Sopyan I, Kaur J. (2009) Preparation and characterization of porous hydroxyapatite through polymeric sponge method. , Ceram. Int 35, 3161-3168.
- 239.Bellucci D, Cannillo V, Sola A. (2010) Shell scaffolds: a new approach towards high strength bioceramic scaffolds for bone regeneration. , Mater. Lett 64, 203-206.
- 240.Cunningham E, Dunne N, Walker G, Maggs C, Wilcox R et al.Hydroxyapatite bone substitutes developed via replication of natural marine sponges. , J. Mater. Sci. Mater. Med 2010, 2255-2261.
- 241.J H Sung, K H Shin, Y H Koh, W Y Choi, Jin Y et al.Preparation of the reticulated hydroxyapatite ceramics using carbon-coated polymeric sponge with elongated pores as a novel template. , Ceram. Int 2011, 2591-2596.
- 242.F D Mishima, Louro L H L, F N Moura, L A Gobbo, Silva da et al. (2012) Hydroxyapatite scaffolds produced by hydrothermal deposition of monetite on polyurethane sponges substrates. Key Eng. , Mater 493-494.
- 243.Hannickel A, Silva da, M H P.Novel bioceramic scaffolds for regenerative medicine. , Bioceram. Dev. Appl 2015, 1000082.
- 244.Das S, Kumar S, Doloi B, Bhattacharyya B. (2016) Experimental studies of ultrasonic machining on hydroxyapatite bio-ceramics. , Int. J. Adv. Manuf. Tech 86, 829-839.
- 245.Velayudhan S, Ramesh P, M C Sunny, H K Varma. (2000) Extrusion of hydroxyapatite to clinically significant shapes. , Mater. Lett 46, 142-146.
- 246.H Y Yang, Thompson I, S F Yang, X P Chi, Evans J R G et al. (2008) Dissolution characteristics of extrusion freeformed hydroxyapatite – tricalcium phosphate scaffolds. , J. Mater. Sci. Mater. Med 19, 3345-3353.
- 247.Yang S, Yang H, Chi X, Evans J R G, Thompson I et al. (2008) Rapid prototyping of ceramic lattices for hard tissue scaffolds. 29, 1802-1809.
- 248.H Y Yang, X P Chi, Yang S, Evans J R G.Mechanical strength of extrusion freeformed calcium phosphate filaments. , J. Mater. Sci. Mater. Med 2010, 1503-1510.
- 249.P, L M Atayde, M A Silva, Silva da, P A Fernandes et al.Characterization and preliminary in vivo evaluation of a novel modified hydroxyapatite produced by extrusion and spheronization techniques. , J. Biomed. Mater. Res. B Appl. Biomater 2011, 170-179.
- 250.Lim S, Chun S, Yang D, Kim S. (2012) Comparison study of porous calcium phosphate blocks prepared by piston and screw type extruders for bone scaffold. Tissue Eng. , Regen. Med 9, 51-55.
- 251.D M Blake, Tomovic S, R W Jyung. (2013) Extrusion of hydroxyapatite ossicular prosthesis. Ear Nose Throat J. 92, 490-494.
- 252.A I Muthutantri, Huang J, M J Edirisinghe, Bretcanu O, A R Boccaccini.Dipping and electrospraying for the preparation of hydroxyapatite foams for bone tissue engineering. , Biomed. Mater 25009(14), pages)..
- 253.Roncari E, Galassi C, Pinasco P. (2000) Tape casting of porous hydroxyapatite ceramics. , J. Mater. Sci. Lett 19, 33-35.
- 254.Tian T, Jiang D, Zhang J, Lin Q. (2007) Aqueous tape casting process for hydroxyapatite. , J. Eur. Ceram. Soc 27, 2671-2677.
- 255.Tanimoto Y, Shibata Y, Murakami A, Miyazaki T, Nishiyama N. (2009) Effect of varying HAP/TCP ratios in tape-cast biphasic calcium phosphate ceramics on responcce in. 18, 71-76.
- 256.Tanimoto Y, Teshima M, Nishiyama N, Yamaguchi M, Hirayama S et al.Tape-cast and sintered β-tricalcium phosphate laminates for biomedical applications: effect of milled Al2O3 fiber additives on microstructural and mechanical properties. , J. Biomed. Mater. Res. B Appl. Biomater 2012, 2261-2268.
- 257.Khamkasem C, Chaijaruwanich A. (2013) Effect of binder/plasticizer ratios in aqueous-based tape casting on mechanical properties of bovine hydroxyapatite tape. Ferroelectrics. 455-129.
- 258.Suzuki S, Itoh K, Ohgaki M, Ohtani M, Ozawa M. (1999) Preparation of sintered filter for ion exchange by a doctor blade method with aqueous slurries of needlelike hydroxyapatite. , Ceram. Int 25, 287-291.
- 259.Nishikawa H, Hatanaka R, Kusunoki M, Hayami T, Hontsu S. (2008) Preparation of freestanding hydroxyapatite membranes excellent biocompatibility and flexibility. , Appl. Phys. Express 1, 088001.
- 260.Padilla S, Roman J, Vallet-Regí M. (2002) Synthesis of porous hydroxyapatites by combination of gel casting and foams burn out methods. , J. Mater. Sci. Mater. Med 13, 1193-1197.
- 261.T Y Yang, J M Lee, S Y Yoon, H C Park.Hydroxyapatite scaffolds processed using a TBA-based freeze-gel casting/polymer sponge technique. , J. Mater. Sci. Mater. Med 2010, 1495-1502.
- 262.Baradararan S, Hamdi M, I H Metselaar.Biphasic calcium phosphate (BCP) macroporous scaffold with different ratios of HA/β-TCP by combination of gel casting and polymer sponge methods. , Adv. Appl. Ceram 2012, 367-373.
- 263.Inoue K, Sassa K, Yokogawa Y, Sakka Y, Okido M et al. (2003) Control of crystal orientation of hydroxyapatite by imposition of a high magnetic field. , Mater. Trans 44, 1133-1137.
- 264.Iwai K, Akiyama J, Tanase T, Asai S. (2007) Alignment of HAp crystal using a sample rotation in a static magnetic field. , Mater. Sci. Forum 1, 539-543.
- 265.Iwai K, Akiyama J, Asai S. (2007) Structure control of hydroxyapatite using a magnetic field. , Part, Mater. Sci. Forum 2, 561-565.
- 266.Sakka Y, Takahashi K, T S Suzuki, Ito S, Matsuda N. (2008) Texture development of hydroxyapatite ceramics by colloidal processing in a high magnetic field followed by sintering. , Mater. Sci. Eng. A 475-27.
- 268.Kang J, Hadfield M. (2001) Parameter optimization by Taguchi methods for finishing advanced ceramic balls using a novel eccentric lapping machine. , Proc. Inst. Mech. Eng. B 215-69.
- 269.S, Yong Y, M J Rys, Lei S. (2013) Machining assessment of nano-crystalline hydroxyapatite bio-ceramic. , J. Manuf. Processes 15, 666-672.
- 270.Kurella A, N B Dahotre. (2005) Surface modification for bioimplants: the role of laser surface engineering. , J. Biomater. Appl 20, 5-50.
- 271.F N Oktar, Genc Y, Goller G, E Z, L S Ozyegin et al. (2004) Sintering of synthetic hydroxyapatite compacts. Key Eng. , Mater 264-268.
- 272.Georgiou G, J C Knowles, J E Barralet. (2004) Dynamic shrinkage behavior of hydroxyapatite and glass-reinforced hydroxyapatites. , J. Mater. Sci 39, 2205-2208.
- 273.B H Fellah, Layrolle P. (2009) Sol-gel synthesis and characterization of macroporous calcium phosphate bioceramics containing microporosity. Acta Biomater. 5, 735-742.
- 274.Dudek A, Kolan C. (2010) Assessments of shrinkage degree in bioceramic sinters HA+ZrO2. Diffusion and Defect Data B: Solid State Phenomena. 165-25.
- 275.Ayed Ben, Bouaziz F, Bouzouita J, K. (2000) Pressureless sintering of fluorapatite under oxygen atmosphere. , J. Eur. Ceram. Soc 20, 1069-1076.
- 276.He Z, Ma J, Wang C. (2005) Constitutive modeling of the densification and the grain growth of hydroxyapatite ceramics. Biomaterials. 26, 1613-1621.
- 278.E A Monroe, Votava W, D B Bass, McMullen J. (1971) New calcium phosphate ceramic material for bone and tooth. 50, 860-861.
- 279.Landi E, Tampieri A, Celotti G, Sprio S. (2000) Densification behaviour and mechanisms of synthetic hydroxyapatites. , J. Eur. Ceram. Soc 20, 2377-2387.
- 280.Chen S, Wang W, Kono H, Sassa K, Asai S. (2010) Abnormal grain growth of hydroxyapatite ceramic sintered in a high magnetic field. , J. Cryst. Growth 312-323.
- 281.A J Ruys, Wei M, C, M R Dickson, Brandwood A et al. (1995) Sintering effect on the strength of hydroxyapatite. Biomaterials. 16, 409-415.
- 282.P van Landuyt, Li F, J P Keustermans, J M Streydio, Delannay F et al. (1995) The influence of high sintering temperatures on the mechanical properties of hydroxylapatite. , J. Mater. Sci. Mater. Med 6, 8-13.
- 283.Pramanik S, A K, K N Rai, Garg A. (2007) Development of high strength hydroxyapatite by solid-state-sintering process. , Ceram. Int 33, 419-426.
- 284.Haberko K, M, Brzezińska-Miecznik J, Haberko M, Mozgawa W et al. (2006) Natural hydroxyapatite – its behaviour during heat treatment. , J. Eur. Ceram. Soc 26, 537-542.
- 285.Haberko K, M, Mozgawa W, Pyda A, Brzezińska-Miecznik J et al. (2009) Behaviour of bone origin hydroxyapatite at elevated temperatures and. in O2 and CO2 atmospheres. Ceram. Int 35, 2537-2540.
- 286.A M Janus, Faryna M, Haberko K, Rakowska A, Panz T. (2008) Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones. , Mikrochim. Acta 161-349.
- 287.M E Bahrololoom, Javidi M, Javadpour S, Ma J. (2009) Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. , J. Ceram. Process. Res 10, 129-138.
- 288.Mostafa N Y Characterization. (2005) thermal stability and sintering of hydroxyapatite powders prepared by different routes. , Mater. Chem. Phys 94, 333-341.
- 289.Suchanek W, Yashima M, Kakihana M, Yoshimura M. (1997) Hydroxyapatite ceramics with selected sintering additives. Biomaterials. 18, 923-933.
- 290.S J Kalita, Bose S, Bandyopadhyay A, H L. (2003) Oxide based sintering additives for HAp ceramics. , Ceram. Trans 147-63.
- 291.S J Kalita, Bose S, H L, Bandyopadhyay A. (2004) CaO–P2O5–Na2O-based sintering additives for hydroxyapatite (HAp) ceramics. Biomaterials. 25, 2331-2339.
- 292.T V Safronova, V I Putlyaev, M A Shekhirev, Y D Tretyakov, A V Kuznetsov et al. (2009) Densification additives for hydroxyapatite ceramics. , J. Eur. Ceram. Soc 29, 1925-1932.
- 293.Muralithran G, Ramesh S. (2000) Effects of sintering temperature on the properties of hydroxyapatite. , Ceram. Int 26, 221-230.
- 294.Eskandari A, Aminzare M, Hassani H, Barounian H, Hesaraki S et al.Densification behavior and mechanical properties of biomimetic apatite nanocrystals. , Curr. Nanosci 2011, 776-780.
- 295.Ramesh S, Tolouei R, C Y Tan, K L Aw, W H Yeo et al. (2011) Sintering of hydroxyapatite ceramic produced by wet chemical method. , Adv. Mater. Res 264-265.
- 296.S F Ou, S Y Chiou, K L Ou. (2013) Phase transformation on hydroxyapatite decomposition. , Ceram. Int 39, 3809-3816.
- 297.Bernache-Assollant D, Ababou A, Champion E, Heughebaert M. (2003) Sintering of calcium phosphate hydroxyapatite Ca10(PO4)6(OH)2 I. Calcination and particle growth. , J. Eur. Ceram. Soc 23, 229-241.
- 298.Ramesh S, C Y Tan, S B Bhaduri, W D Teng, Sopyan I. (2008) Densification behaviour of nanocrystalline hydroxyapatite bioceramics. , J. Mater. Process. Technol 206-221.
- 299.Wang J, L. (2010) Grain-size dependence of the hardness of submicrometer and nanometer hydroxyapatite. , J. Am. Ceram. Soc 93, 601-604.
- 300.Kobayashi S, Kawai W, Wakayama S. (2006) The effect of pressure during sintering on the strength and the fracture toughness of hydroxyapatite ceramics. , J. Mater. Sci. Mater. Med 17, 1089-1093.
- 301.I W Chen, X H Wang. (2000) Sintering dense nanocrystalline ceramics without final-stage grain growth. , Nature 404-168.
- 302.Mazaheri M, Haghighatzadeh M, A M Zahedi, S K. (2009) Effect of a novel sintering process on mechanical properties of hydroxyapatite ceramics. , J. Alloys Compd 471-180.
- 303.Lin K, Chen L, Chang J.Fabrication of dense hydroxyapatite nanobioceramics with enhanced mechanical properties via two-step sintering process. , Int. J. Appl. Ceram. Technol 2012, 479-485.
- 304.Panyata S, Eitssayeam S, Rujijanagul G, Tunkasiri T, Pengpat K.Property development of hydroxyapatite ceramics by two-step sintering. , Adv. Mater. Res 2012, 190-193.
- 305.Esnaashary M, Fathi M, Ahmadian M.The effect of the two-step sintering process on consolidation of fluoridated hydroxyapatite and its mechanical properties and bioactivity. , Int. J. Appl. Ceram. Technol 2014, 47-56.
- 306.Feng P, Niu M, Gao C, Peng S, Shuai C. (2014) A novel two-step sintering for nano-hydroxyapatite scaffolds for bone tissue engineering. Sci. Report 4, 5599.
- 307.Halouani R, Bernache-Assolant D, Champion E, Ababou A. (1994) Microstructure and related mechanical properties of hot pressed hydroxyapatite ceramics. , J. Mater. Sci. Mater. Med 5, 563-568.
- 308.Kasuga T, Ota Y, Tsuji K, Abe Y. (1996) Preparation of high-strength calcium phosphate ceramics with low modulus of elasticity containing β-Ca(PO3)2 fibers. , J. Am. Ceram. Soc 79, 1821-1824.
- 309.W L Suchanek, Yoshimura M. (1998) Preparation of fibrous, porous hydroxyapatite ceramics from hydroxyapatite whiskers. , J. Am. Ceram. Soc 81, 765-767.
- 310.Kim Y, S R Kim, Song H, Yoon H. (2005) Preparation of porous hydroxyapatite/TCP composite block using a hydrothermal hot pressing method. , Mater. Sci. Forum 486-487.
- 311.J G Li, Hashida T. (2006) In situ formation of hydroxyapatite-whisker ceramics by hydrothermal hot-pressing method. , J. Am. Ceram. Soc 89, 3544-3546.
- 312.J G Li, Hashida T. (2007) Preparation of hydroxyapatite ceramics by hydrothermal hot-pressing method at 300. 42, 5013-5019.
- 313.N V Petrakova, A S Lysenkov, A, A, A Y Fedotov et al.Effect of hot pressing temperature on the microstructure and strength of hydroxyapatite ceramic. , Inorg. Mater. Appl. Res 2013, 362-367.
- 314.Nakahira A, Murakami T, Onoki T, Hashida T, Hosoi K. (2005) Fabrication of porous hydroxyapatite using hydrothermal hot pressing and post-sintering. , J. Am. Ceram. Soc 88, 1334-1336.
- 315.M A Auger, Savoini B, Muñoz A, Leguey T, M A et al. (2009) Mechanical characteristics of porous hydroxyapatite/oxide composites produced by post-sintering hot isostatic pressing. , Ceram. Int 35, 2373-2380.
- 316.C, Graça M P F, Sombra A S B, M A Valente. (2009) Structural and electrical study of calcium phosphate obtained by a microwave radiation assisted procedure. , Phys. Rev. B Condens. Matter 404-1503.
- 317.Chanda A, Dasgupta S, Bose S, Bandyopadhyay A. (2009) Microwave sintering of calcium phosphate ceramics. , Mater. Sci. Eng. C 29, 1144-1149.
- 318.Veljović D, Zalite I, Palcevskis E, Smiciklas I, Petrović R et al. (2010) . Microwave sintering of fine grained HAP and HAP/TCP bioceramics. Ceram. Int 36, 595-603.
- 319.S J Kalita, Verma S. (2010) Nanocrystalline hydroxyapatite bioceramic using microwave radiation: synthesis and characterization. , Mater. Sci. Eng. C 30, 295-303.
- 320.Veljović D, Palcevskis E, Dindune A, Putić S, Balać I et al.Microwave sintering improves the mechanical properties of biphasic calcium phosphates from hydroxyapatite microspheres produced from hydrothermal processing. , J. Mater. Sci 2010, 3175-3183.
- 321.Wu Q, Zhang X, Wu B, Huang W.Effects of microwave sintering on the properties of porous hydroxyapatite scaffolds. , Ceram. Int 2013, 2389-2395.
- 322.Tarafder S, V K Balla, N M Davies, Bandyopadhyay A, Bose S.Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. , J. Tissue Eng. Regen. Med 2013, 631-641.
- 323.Thuault A, Savary E, J C Hornez, Moreau G, Descamps M et al.Improvement of the hydroxyapatite mechanical properties by direct microwave sintering in single mode cavity. , J. Eur. Ceram. Soc 2014, 1865-1871.
- 324.Tovstonoh H, Sych O, Skorokhod V.Effect of microwave sintering temperature on structure and properties of bioceramics based on biogenic hydroxyapatite. , Funct. Mater 2014, 487-491.
- 325.Tarafder S, W S Dernell, Bandyopadhyay A, S.and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: mechanical properties and in vivo osteogenesis in a rabbit model. , J. Biomed. Mater. Res. B Appl. Biomater 2015, 679-690.
- 326.Nakamura T, Fukuhara T, Izui H. (2006) Mechanical properties of hydroxyapatites sintered by spark plasma sintering. , Ceram. Trans 194-265.
- 327.Zhang F, Lin K, Chang J, Lu J, Ning C. (2008) Spark plasma sintering of macroporous calcium phosphate scaffolds from nanocrystalline powders. , J. Eur. Ceram. Soc 28, 539-545.
- 328.Grossin D, Rollin-Martinet S, Estournès C, Rossignol F, Champion E et al. (2010) Biomimetic apatite sintered at very low temperature by spark plasma sintering: physico-chemistry and microstructure aspects. Acta Biomater. 6, 577-585.
- 329.Chesnaud A, Bogicevic C, Karolak F, Estournes C, Dezanneau G. (2007) Preparation of transparent oxyapatite ceramics by combined use of freeze-drying and spark-plasma sintering. , Chem. Comm 1550-1552.
- 330.Eriksson M, Liu Y, Hu J, Gao L, Nygren M et al.Transparent hydroxyapatite ceramics with nanograin structure prepared by high pressure spark plasma sintering at the minimized sintering temperature. , J. Eur. Ceram. Soc 2011, 1533-1540.
- 331.Liu Y, Shen Z.Dehydroxylation of hydroxyapatite in dense bulk ceramics sintered by spark plasma sintering. , J. Eur. Ceram. Soc 2012, 2691-2696.
- 332.Yoshida H, B N Kim, H W Son, Y H Han, Kim S. (2013) Superplastic deformation of transparent hydroxyapatite. Scripta Mater. 69, 155-158.
- 333.B N Kim, Prajatelistia E, Y H Han, H W Son, Sakka Y et al. (2013) Transparent hydroxyapatite ceramics consolidated by spark plasma sintering. Scripta Mater. 69, 366-369.
- 334.Yun J, Son H, Prajatelistia E, Y H Han, Kim S et al.Characterisation of transparent hydroxyapatite nanoceramics prepared by spark plasma sintering. , Adv. Appl. Ceram 2014, 67-72.
- 335.Li Z, K A. (2015) Transparent hydroxyapatite obtained through spark plasma sintering: optical and mechanical properties. , Key Eng. Mater 631-51.
- 336.Yanagisawa K, J H Kim, Sakata C, Onda A, Sasabe E et al. (2010) Hydrothermal sintering under mild temperature conditions: preparation of calcium-deficient hydroxyapatite compacts. , Z. Naturforsch. B 65, 1038-1044.
- 337.Hosoi K, Hashida T, Takahashi H, Yamasaki N, Korenaga T. (1996) New processing technique for hydroxyapatite ceramics by the hydrothermal hot-pressing method. , J. Am. Ceram. Soc 79, 2771-2774.
- 338.K A Gross, C. (2002) Biomedical application of apatites. In: Phosphates: geochemical, geobiological and materials importance. Series: Reviews. in Mineralogy and Geochemistry , Washington, D.C., USA 48, 631-672.
- 342.Black J. (2005) Biological performance of materials: fundamentals of biocompatibility. Fourth Ed. , Boca Raton, FL, USA 520.
- 343.C B, M G Norton. (2013) Ceramic materials: science and engineering. 2nd ed. , New York, USA 766.
- 344.Benaqqa C, Chevalier J, Saädaoui M, Fantozzi G. (2005) Slow crack growth behaviour of hydroxyapatite ceramics. Biomaterials. 26, 6106-6112.
- 345.Pecqueux F, Tancret F, Payraudeau N, J M Bouler.Influence of microporosity and macroporosity on the mechanical properties of biphasic calcium phosphate bioceramics: modelling and experiment. , J. Eur. Ceram. Soc 2010, 819-829.
- 346.Ramesh S, C Y Tan, Sopyan I, Hamdi M, W D Teng.Consolidation of nanocrystalline hydroxyapatite powder. , Sci. Technol. Adv. Mater 2007, 124-130.
- 347.Johnson Wagoner, A J Herschler, A B. (2011) A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 7, 16-30.
- 348.W L Suchanek, Yoshimura M. (1998) Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. , J. Mater. Res 13, 94-117.
- 349.Fan X, E D Case, Ren F, Shu Y, M J Baumann.Part I: Porosity dependence of the Weibull modulus for hydroxyapatite and other brittle materials. , J. Mech. Behav. Biomed. Mater 2012, 21-36.
- 350.Fan X, E D Case, Gheorghita I, M J Baumann.Weibull modulus and fracture strength of highly porous hydroxyapatite. , J. Mech. Behav. Biomed. Mater 2013, 283-295.
- 351.Cordell J, Vogl M, Johnson A.The influence of micropore size on the mechanical properties of bulk hydroxyapatite and hydroxyapatite scaffolds. , J. Mech. Behav. Biomed. Mater 2009, 560-570.
- 352.Suzuki S, Sakamura M, Ichiyanagi M, Ozawa M. (2004) Internal friction of hydroxyapatite and fluorapatite. , Ceram. Int 30, 625-627.
- 353.Suzuki S, Takahiro K, Ozawa M. (1998) Internal friction and dynamic modulus of polycrystalline ceramics prepared from stoichiometric and Ca-deficient hydroxyapatites. , Mater. Sci. Eng. B 55, 68-70.
- 354.J M Bouler, Trecant M, Delecrin J, Royer J, Passuti N et al. (1996) Macroporous biphasic calcium phosphate ceramics: influence of five synthesis parameters on compressive. 32, 603-609.
- 355.Tancret F, J M Bouler, Chamousset J, L M Minois. (2006) Modelling the mechanical properties of microporous and macroporous biphasic calcium phosphate bioceramics. , J. Eur. Ceram. Soc 26, 3647-3656.
- 356.Huec J C le, Schaeverbeke T, Clement D, Faber J, A le Rebeller. (1995) Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials. 16, 113-118.
- 357.Y H Hsu, I G Turner, A W Miles. (2009) Mechanical properties of three different compositions of calcium phosphate bioceramic following immersion in Ringer’s solution and distilled. 20, 2367-2374.
- 358.A M Torgalkar. (1979) Resonance frequency technique to determine elastic modulus of hydroxyapatite. , J. Biomed. Mater. Res 13, 907-920.
- 360.Fan X, E D Case, Ren F, Shu Y, M J Baumann.Part II: fracture strength and elastic modulus as a function of porosity for hydroxyapatite and other brittle materials. , J. Mech. Behav. Biomed. Mater 2012, 99-110.
- 361.Aza P N de, Aza A H de, S de Aza. (2005) Crystalline bioceramic materials. , Bol. Soc. Esp. Ceram. V 44, 135-145.
- 362.Fritsch A, Dormieux L, Hellmich C, Sanahuja J. (2009) Mechanical behavior of hydroxyapatite biomaterials: an experimentally validated micromechanical model for elasticity and strength. , J. Biomed. Mater. Res. A 88-149.
- 363.W Y Ching, Rulis P, Misra A. (2009) Ab initio elastic properties and tensile strength of crystalline hydroxyapatite. Acta Biomater. 5, 3067-3075.
- 364.Fritsch A, Hellmich C, Dormieux L. (2010) The role of disc-type crystal shape for micromechanical predictions of elasticity and strength of hydroxyapatite biomaterials. , Philos. Trans. R. Soc. Lond. A 368-1913.
- 365.Menéndez-Proupin E, Cervantes-Rodríguez S, Osorio-Pulgar R, Franco-Cisterna M, Camacho-Montes H et al.Computer simulation of elastic constants of hydroxyapatite and fluorapatite. , J. Mech. Behav. Biomed. Mater 2011, 1011-1120.
- 366.J P Sun, Song Y, G W Wen, Wang Y, Yang R. (2013) Softening of hydroxyapatite by vacancies: a first principles investigation. , Mater. Sci. Eng. C 33, 1109-1115.
- 367.M C Sha, Li Z, R C Bradt. (1994) Single-crystal elastic constants of fluorapatite. , Ca5F(PO4)3. J. Appl. Phys 75, 7784-7787.
- 368.Wakai F, Kodama Y, Sakaguchi S, Nonami T. (1990) Superplasticity of hot isostatically pressed hydroxyapatite. , J. Am. Ceram. Soc 73, 457-460.
- 369.Tago K, Itatani K, T S Suzuki, Sakka Y, Koda S. (2005) Densification and superplasticity of hydroxyapatite ceramics. , J. Ceram. Soc. Jpn 113-669.
- 370.E L Burger, Patel V. (2007) Calcium phosphates as bone graft extenders. Orthopedics. 30, 939-942.
- 371.L M Rodriguez-, Vallet-Regí M, Ferreira J M F, M P Ginebra, Aparicio C et al. (2002) A hydroxyapatite ceramic bodies with tailored mechanical properties for different applications. , J. Biomed. Mater. Res 60, 159-166.
- 372.Song J, Liu Y, Zhang Y, Jiao L. (2011) Mechanical properties of hydroxyapatite ceramics sintered from powders with different morphologies. , Mater. Sci. Eng. A 528-5421.
- 373.S V Dorozhkin.Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. , J. Funct. Biomater 2015, 708-832.
- 374.Bouslama N, Ayed Ben, Bouaziz F, J. (2009) Sintering and mechanical properties of tricalcium phosphate – fluorapatite composites. , Ceram. Int 35, 1909-1917.
- 375.Suchanek W, Yashima M, Kakihana M, Yoshimura M. (1996) Processing and mechanical properties of hydroxyapatite reinforced with hydroxyapatite whiskers. Biomaterials. 17, 1715-1723.
- 376.Suchanek W, Yashima M, Kakihana M, Yoshimura M. (1997) Hydroxyapatite/hydroxyapatite-whisker composites without sintering additives: mechanical properties and microstructural evolution. , J. Am. Ceram. Soc 80, 2805-2813.
- 377.Simsek D, Ciftcioglu R, Guden M, Ciftcioglu M, Harsa S. (2004) Mechanical properties of hydroxyapatite composites reinforced with hydroxyapatite whiskers. Key Eng. , Mater 264-268.
- 378.Bose S, Banerjee A, Dasgupta S, A Synthesis Bandyopadhyay. (2009) processing, mechanical, and biological property characterization of hydroxyapatite whisker-reinforced hydroxyapatite composites. , J. Am. Ceram. Soc 92, 323-330.
- 379.Lie-Feng L, Xiao-Yi H, Y X Cai, Weng J.Reinforcing of porous hydroxyapatite ceramics with hydroxyapatite fibres for enhanced bone tissue engineering. , J. Biomim. Biomater. Tissue Eng 2011, 67-73.
- 380.Shiota T, Shibata M, Yasuda K, Matsuo Y. (2009) Influence of β-tricalcium phosphate dispersion on mechanical properties of hydroxyapatite ceramics. , J. Ceram. Soc. Jpn 116-1002.
- 381.Shuai C, Feng P, Nie Y, Hu H, Liu J et al.Nano-hydroxyapatite improves the properties of β-tricalcium phosphate bone scaffolds. , Int. J. Appl. Ceram. Technol 2013, 1003-1013.
- 382.S V Dorozhkin, Ajaal T. (2009) Toughening of porous bioceramic scaffolds by bioresorbable polymeric coatings. , Proc. Inst. Mech. Eng. Η 223-459.
- 383.S V Dorozhkin, Ajaal T.Strengthening of dense bioceramic samples using bioresorbable polymers – a statistical approach. , J. Biomim. Biomater. Tissue Eng 2009, 27-39.
- 384.Dressler M, Dombrowski F, Simon U, Börnstein J, V D Hodoroaba et al.Influence of gelatin coatings on compressive strength of porous hydroxyapatite ceramics. , J. Eur. Ceram. Soc 2011, 523-529.
- 385.F J Martinez-Vazquez, F H Perera, Miranda P, Pajares A, Guiberteau F. (2010) Improving the compressive strength of bioceramic robocast scaffolds by polymer infiltration. Acta Biomater. 6, 4361-4368.
- 386.A Y Fedotov, N V Bakunova, V S Komlev, S M Barinov. (2011) High-porous calcium phosphate bioceramics reinforced by chitosan infiltration. , Dokl. Chem 439-233.
- 387.F J Martínez-Vázquez, Pajares A, Guiberteau F, Miranda P. (2014) Effect of polymer infiltration on the flexural behavior of β-tricalcium phosphate robocast scaffolds. Materials. 7, 4001-4018.
- 388.L H He, O C Standard, T, B A Latella, M V Swain. (2008) Mechanical behaviour of porous hydroxyapatite. Acta Biomater. 4, 577-586.
- 389.D C Tancred, B A McCormack, A J Carr. (1998) A synthetic bone implant macroscopically identical to cancellous bone. Biomaterials. 19, 2303-2311.
- 391.Schliephake H, F W Neukam, Klosa D. (1991) Influence of pore dimensions on bone ingrowth into porous hydroxylapatite blocks used as bone graft substitutes. A histometric study. , Int. J. Oral Maxillofac. Surg 20, 53-58.
- 392.Otsuki B, Takemoto M, Fujibayashi S, Neo M, Kokubo T et al. (2006) Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 27, 5892-5900.
- 393.K A Hing, S M Best, Bonfield W. (1999) Characterization of porous hydroxyapatite. , J. Mater. Sci. Mater. Med 10, 135-145.
- 394.J X Lu, Flautre B, Anselme K, Hardouin P, Gallur A et al. (1999) Role of interconnections in porous bioceramics on bone recolonization in vitro and in. 10, 111-120.
- 395.A C Jones, C H Arns, A P Sheppard, D W Hutmacher, B K Milthorpe et al. (2007) Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials. 28, 2491-2504.
- 396.Tamai N, Myoui A, Kudawara I, Ueda T, Yoshikawa H. (2010) Novel fully interconnected porous hydroxyapatite ceramic in surgical treatment of benign bone tumor. , J. Orthop. Sci 15, 560-568.
- 397.Sakane M, Tsukanishi T, Funayama T, Kobayashi M, Ochiai N. (2012) Unidirectional porous β-tricalcium phosphate bone substitute: examination of balance between new bone formation and absorption. 493-494.
- 398.Panzavolta S, Torricelli P, Amadori S, Parrilli A, Rubini K et al. (2013) 3D interconnected porous biomimetic scaffolds: in vitro cell response. , J. Biomed. Mater. Res. A 101-3560.
- 399.Jin L, Z Q Feng, Wang T, Ren Z, Ma S et al. (2014) A novel fluffy hydroxylapatite fiber scaffold with deep interconnected pores designed for three-dimensional cell culture. , J. Mater. Chem. B 2, 129-136.
- 400.Flautre B, Descamps M, Delecourt C, M C Blary, Hardouin P. (2001) Porous HA ceramic for bone replacement: role of the pores and interconnections – experimental study in the rabbits. , J. Mater. Sci. Mater. Med 12, 679-682.
- 401.Tamai N, Myoui A, Tomita T, Nakase T, Tanaka J et al. (2002) Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in. 59, 110-117.
- 402.Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F et al. (2006) Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials. 27, 3230-3237.
- 403.Okamoto M, Dohi Y, Ohgushi H, Shimaoka H, Ikeuchi M et al. (2006) Influence of the porosity of hydroxyapatite ceramics on in vitro and in vivo bone formation by cultured rat bone marrow stromal cells. , J. Mater. Sci. Mater. Med 17, 327-336.
- 404.Zhang L, Hanagata N, Maeda M, Minowa T, Ikoma T et al. (2009) Porous hydroxyapatite and biphasic calcium phosphate ceramics promote ectopic osteoblast differentiation from mesenchymal stem cells. , Sci. Technol. Adv. Mater 025003(9), pages)..
- 405.Li X, Liu H, Niu X, Fan Y, Feng Q et al.Osteogenic differentiation of human adipose-derived stem cells induced by osteoinductive calcium phosphate ceramics. , J. Biomed. Mater. Res. B Appl. Biomater 2011, 10-19.
- 406.M H Hong, S M Kim, M H Han, Y H Kim, Y K Lee et al.Evaluation of microstructure effect of the porous spherical β-tricalcium phosphate granules on cellular responses. , Ceram. Int 2014, 6095-6102.
- 407.Godoy R F de, Hutchens S, Campion C, Blunn G.Silicate-substituted calcium phosphate with enhanced strut porosity stimulates osteogenic differentiation of human mesenchymal stem cells. , J. Mater. Sci. Mater. Med 54(12), pages)..
- 408.Omae H, Mochizuki Y, Yokoya S, Adachi N, Ochi M. (2006) Effects of interconnecting porous structure of hydroxyapatite ceramics on interface between grafted tendon and ceramics. , J. Biomed. Mater. Res. A 79-329.
- 409.Yoshikawa H, Tamai N, Murase T, Myoui A. (2009) Interconnected porous hydroxyapatite ceramics for bone tissue engineering. , J. R. Soc. Interface 6, 341-348.
- 410.Ribeiro G B M, R M Trommer, Santos dos, L A Bergmann, P C. (2011) Novel method to produce β-TCP scaffolds. , Mater. Lett 65, 275-277.
- 411.Silva T S N, B T Primo, Jr Silva, A N Machado, D C Viezzer et al. (2011) Use of calcium phosphate cement scaffolds for bone tissue engineering: in vitro study. Acta Cir. , Bras 26, 7-11.
- 412.de Moraes MacHado, J L Giehl, I C Nardi, B dos Santos N, A L.Evaluation of scaffolds based on α-tricalcium phosphate cements for tissue engineering applications. , IEEE Trans. Biomed. Eng 2011, 1814-1819.
- 413.S H Li, Wijn J R de, Layrolle P, K de Groot. (2003) Novel method to manufacture porous hydroxyapatite by dual-phase mixing. , J. Am. Ceram. Soc 86, 65-72.
- 414.Oliveira J F de, Aguiar P F de, A M Rossi, Soares G D A. (2003) Effect of process parameters on the characteristics of porous calcium phosphate ceramics for bone tissue scaffolds. , Artif. Organs 27, 406-411.
- 415.S K, Bhattacharyya S. (2013) Preparation of high strength macroporous hydroxyapatite scaffold. , Mater. Sci. Eng. C 33, 67-71.
- 416.Maeda H, Kasuga T, Nogami M, Kagami H, Hata K et al. (2004) Preparation of bonelike apatite composite sponge. Key Eng. , Mater 254-256.
- 417.Ray A M le, Gautier H, J M Bouler, Weiss P, Merle C.A new technological procedure using sucrose as porogen compound to manufacture porous biphasic calcium phosphate ceramics of appropriate micro- and macrostructure. , Ceram. Int 2010, 93-101.
- 418.S H Li, Wijn J R de, Layrolle P, K de Groot. (2002) Synthesis of macroporous hydroxyapatite scaffolds for bone tissue engineering. , J. Biomed. Mater. Res 61, 109-120.
- 419.Hesaraki S, Sharifi D. (2007) Investigation of an effervescent additive as porogenic agent for bone cement macroporosity. , Biomed. Mater. Eng 17, 29-38.
- 420.Hesaraki S, Moztarzadeh F, Sharifi D. (2007) Formation of interconnected macropores in apatitic calcium phosphate bone cement with the use of an effervescent additive. , J. Biomed. Mater. Res. A 83-80.
- 421.Pal K, Pal S. (2006) Development of porous hydroxyapatite scaffolds. , Mater. Manuf. Process 21, 325-328.
- 422.A C Tas. (2008) Preparation of porous apatite granules from calcium phosphate cement. , J. Mater. Sci. Mater. Med 19, 2231-2239.
- 423.Yao X, Tan S, Jiang D. (2005) Improving the properties of porous hydroxyapatite ceramics by fabricating methods. , J. Mater. Sci 40, 4939-4942.
- 424.H Y Song, M H Youn, Y H Kim, Y K Min, H M Yang et al. (2007) Fabrication of porous β-TCP bone graft substitutes using PMMA powder and their biocompatibility study. , Korean 17, 318-322.
- 425.M H Youn, R K Paul, H Y Song, B T Lee. (2007) Fabrication of porous structure of BCP sintered bodies using microwave assisted synthesized HAp nano powder. , Mater. Sci. Forum 534-536.
- 426.Almirall A, Larrecq G, J A Delgado, Martínez S, M P Ginebra et al. (2004) Fabrication of low temperature hydroxyapatite foams. Key Eng. , Mater 254-256.
- 427.Almirall A, Larrecq G, J A Delgado, Martínez S, J A Planell et al. (2004) Fabrication of low temperature macroporous hydroxyapatite scaffolds by foaming and hydrolysis of an α-TCP paste. Biomaterials. 25, 3671-3680.
- 428.Huang X, Miao X. (2007) Novel porous hydroxyapatite prepared by combining H2O2 foaming with PU sponge and modified with PLGA and bioactive. 21, 351-374.
- 429.Strnadova M, Protivinsky J, Strnad J, Vejsicka Z. (2008) Preparation of porous synthetic nanostructured HA scaffold. Key Eng. , Mater 361-363.
- 430.Li B, Chen X, Guo B, Wang X, Fan H et al. (2009) Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomater. 5, 134-143.
- 431.Cheng Z, Zhao K, Z P Wu. (2015) Structure control of hydroxyapatite ceramics via an electric field assisted freeze casting method. , Ceram. Int 41, 8599-8604.
- 432.Takagi S, L C Chow. (2001) Formation of macropores in calcium phosphate cement implants. , J. Biomed. Mater. Res 12, 135-139.
- 433.Walsh D, Tanaka J. (2001) Preparation of a bone-like apatite foam cement. , J. Mater. Sci. Mater. Med 12, 339-344.
- 434.Tadic D, Beckmann F, Schwarz K, Epple M. (2004) A novel method to produce hydroxylapatite objects with interconnecting porosity that avoids sintering. Biomaterials. 25, 3335-3340.
- 435.V S Komlev, S M Barinov. (2002) Porous hydroxyapatite ceramics of bi-modal pore size distribution. , J. Mater. Sci. Mater. Med 13, 295-299.
- 436.Sepulveda P, J G Binner, S O Rogero, O Z Higa, J C Bressiani. (2000) Production of porous hydroxyapatite by the gel-casting of foams and cytotoxic. 50, 27-34.
- 437.Y H Hsu, I G Turner, A W Miles. (2007) Mechanical characterization of dense calcium phosphate bioceramics with interconnected porosity. , J. Mater. Sci. Mater. Med 18, 2319-2329.
- 438.H G Zhang, Zhu Q. (2007) Preparation of porous hydroxyapatite with interconnected pore architecture. , J. Mater. Sci. Mater. Med 18, 1825-1829.
- 439.Chevalier E, Chulia D, Pouget C, Viana M. (2008) Fabrication of porous substrates: a review of processes using pore forming agents in the biomaterial. 97, 1135-1154.
- 440.Y J Tang, Y F Tang, C T Lv, Z H. (2008) Preparation of uniform porous hydroxyapatite biomaterials by a new method. , Appl. Surf. Sci 254-5359.
- 441.S T Abdulqader, I A Rahman, Ismail H, Kannan Ponnuraj, Mahmood T et al.A simple pathway in preparation of controlled porosity of biphasic calcium phosphate scaffold for dentin regeneration. , Ceram. Int 2013, 2375-2381.
- 443.F H Wen, Wang F, Gai Y, M T Wang, Q H Lai.Preparation of mesoporous hydroxylapatite ceramics using polystyrene microspheres as template. , Appl. Mech. Mater 2013, 194-198.
- 444.Guda T, Appleford M, Oh S, J L Ong. (2008) A cellular perspective to bioceramic scaffolds for bone tissue engineering: the state of the art. , Curr. Top. Med. Chem 8, 290-299.
- 445.Habraken W J E M, Wolke J G C, J A. (2007) Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev 59, 234-248.
- 447.S K, Bhattacharyya S, Sarkar D. (2011) Preparation of porous scaffold from hydroxyapatite powders. , Mater. Sci. Eng. C 31, 1240-1244.
- 448.Zhao K, Y F Tang, Y S Qin, D F Luo.Polymer template fabrication of porous hydroxyapatite scaffolds with interconnected spherical pores. , J. Eur. Ceram. Soc 2011, 225-229.
- 449.J H Sung, K H Shin, Y W Moon, Y H Koh, W Y Choi et al.Production of porous calcium phosphate (CaP) ceramics with highly elongated pores using carbon-coated polymeric templates. , Ceram. Int 2012, 93-97.
- 450.D S Oha, Y H Kim, Ganbat D, M H Han, Lim P et al.Bone marrow absorption and retention properties of engineered scaffolds with micro-channels and nano-pores for tissue engineering: a proof of concept. , Ceram. Int 2013, 8401-8410.
- 451.Deville S, Saiz E, A P Tomsia. (2006) Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials. 27, 5480-5489.
- 452.E J Lee, Y H Koh, B H Yoon, H E Kim, H W Kim. (2007) Highly porous hydroxyapatite bioceramics with interconnected pore channels using camphene-based freeze casting. , Mater. Lett 61, 2270-2273.
- 453.Fu Q, M N Rahaman, Dogan F, B S. (2008) Freeze casting of porous hydroxyapatite scaffolds. I. Processing and general microstructure. , J. Biomed. Mater. Res. B Appl. Biomater 86, 125-135.
- 454.Impens S, Schelstraete R, Luyten J, Mullens S, Thijs I et al. (2009) Production and characterisation of porous calcium phosphate structures with controllable hydroxyapatite/β-tricalcium phosphate ratios. , Adv. Appl. Ceram 108-494.
- 455.Macchetta A, I G Turner, C R Bowen. (2009) Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-based freeze-casting method. Acta Biomater. 5, 1319-1327.
- 456.Potoczek M, Zima A, Paszkiewicz Z, Ślósarczyk A. (2009) Manufacturing of highly porous calcium phosphate bioceramics via gel-casting using agarose. , Ceram. Int 35, 2249-2254.
- 457.K H Zuo, Y P Zeng, Jiang D. (2010) Effect of polyvinyl alcohol additive on the pore structure and morphology of the freeze-cast hydroxyapatite ceramics. , Mater. Sci. Eng. C 30, 283-287.
- 458.Y M Soon, K H Shin, Y H Koh, J H Lee, W Y Choi et al.Fabrication and compressive strength of porous hydroxyapatite scaffolds with a functionally graded core/shell structure. , J. Eur. Ceram. Soc 2011, 13-18.
- 459.Hesaraki S. (2014) Freeze-casted nanostructured apatite scaffold obtained from low temperature biomineralization of reactive calcium phosphates. Key Eng. , Mater 587-21.
- 460.Ng S, Guo J, Ma J, Loo S C J. (2010) Synthesis of high surface area mesostructured calcium phosphate particles. Acta Biomater. 6, 3772-3781.
- 461.Walsh D, J D Hopwood, Mann S. (1994) Crystal tectonics: construction of reticulated calcium phosphate frameworks in bicontinuous reverse microemulsions. Science. 264-1576.
- 462.Walsh D, Mann S. (1996) Chemical synthesis of microskeletal calcium phosphate in bicontinuous microemulsions. , Chem. Mater 8, 1944-1953.
- 463.Zhao K, Y F Tang, Y S Qin, J Q Wei.Porous hydroxyapatite ceramics by ice templating: freezing characteristics and mechanical properties. , Ceram. Int 2011, 635-639.
- 464.Zhou K, Zhang Y, Zhang D, Zhang X, Li Z et al. (2011) Porous hydroxyapatite ceramics fabricated by an ice-templating method. Scripta Mater. 64, 426-429.
- 465.Flauder S, Gbureck U, F A Muller. (2013) TCP scaffolds with an interconnected and aligned porosity fabricated via ice-templating. Key Eng. , Mater 529-530.
- 466.Zhang Y, Zhou K, Bao Y, Zhang D. (2013) Effects of rheological properties on ice-templated porous hydroxyapatite ceramics. , Mater. Sci. Eng. C 33, 340-346.
- 467.White E, E C Shors. (1986) . Biomaterial aspects of Interpore-200 porous hydroxyapatite. Dent. Clin. North Am 30, 49-67.
- 468.Aizawa M, S F Howell, Itatani K, Yokogawa Y, Nishizawa K et al. (2000) Fabrication of porous ceramics with well-controlled open pores by sintering of fibrous hydroxyapatite particles. , J. Ceram. Soc. Jpn 108-249.
- 469.Nakahira A, Tamai M, Sakamoto K, Yamaguchi S. (2000) Sintering and microstructure of porous hydroxyapatite. , J. Ceram. Soc. Jpn 108-99.
- 470.L M Rodriguez-, Vallet-Regí M, Ferreira J M F. (2002) Fabrication of porous hydroxyapatite bodies by a new direct consolidation method: starch consolidation. , J. Biomed. Mater. Res 60, 232-240.
- 471.Charriere E, Lemaitre J, Zysset P. (2003) Hydroxyapatite cement scaffolds with controlled macroporosity: fabrication protocol and mechanical properties. Biomaterials. 24, 809-817.
- 472.Eichenseer C, Will J, Rampf M, Wend S, Greil P.Biomorphous porous hydroxyapatite-ceramics from rattan (Calamus Rotang). , J. Mater. Sci. Mater. Med 2010, 131-137.
- 473.Zhou L, Wang D, Huang W, Yao A, Kamitakahara M et al. (2009) Preparation and characterization of periodic porous frame of hydroxyapatite. , J. Ceram. Soc. Jpn 117-521.
- 474.Kawata M, Uchida H, Itatani K, Okada I, Koda S et al. (2004) Development of porous ceramics with well-controlled porosities and pore sizes from apatite fibers and their. 15, 817-823.
- 475.Y H Koh, H W Kim, H E Kim, J W Halloran. (2002) Fabrication of macrochannelled-hydroxyapatite bioceramic by a coextrusion process. , J. Am. Ceram. Soc 85, 2578-2580.
- 476.Kitamura M, Ohtsuki C, S I Ogata, Kamitakahara M, Tanihara M et al. (2005) Mesoporous calcium phosphate via post-treatment of α-TCP. , J. Am. Ceram. Soc 88, 822-826.
- 477.Walsh D, Boanini E, Tanaka J, Mann S. (2005) Synthesis of tri-calcium phosphate sponges by interfacial deposition and thermal transformation of self-supporting calcium phosphate films. , J. Mater. Chem 15, 1043-1048.
- 478.Gonzalez-McQuire R, Green D, Walsh D, Hall S, J Y Chane-Ching et al. (2005) Fabrication of hydroxyapatite sponges by dextran sulphate/amino acid templating. Biomaterials. 26, 6652-6656.
- 479.Xu S, Li D, Lu B, Tang Y, Wang C et al. (2007) Fabrication of a calcium phosphate scaffold with a three dimensional channel network and its application to perfusion culture of stem cells. Rapid Prototyping. 13, 99-106.
- 480.Saiz E, Gremillard L, Menendez G, Miranda P, Gryn K et al. (2007) Preparation of porous hydroxyapatite scaffolds. , Mater. Sci. Eng. C 27, 546-550.
- 481.Kamitakahara M, Ohtsuki C, Kawachi G, Wang D, Ioku K. (2008) Preparation of hydroxyapatite porous ceramics with different porous structures using a hydrothermal treatment with different aqueous solutions. , J. Ceram. Soc. Jpn 116-6.
- 482.Peña J, Román J, M V Cabañas, Vallet-Regí M. (2010) An alternative technique to shape scaffolds with hierarchical porosity at physiological temperature. Acta Biomater. 6, 1288-1296.
- 483.Nakamura S, Nakahira A. (2008) Synthesis and evaluation of porous hydroxyapatite prepared by hydrothermal treatment and subsequent sintering method. , J. Ceram. Soc. Jpn 116-42.
- 484.Zhang J, Fujiwara M, Xu Q, Zhu Y, Iwasa M et al. (2008) Synthesis of mesoporous calcium phosphate using hybrid templates. , Micropor. Mesopor. Mater 111-411.
- 485.H Y Song, Islam S, B T Lee. (2008) A novel method to fabricate unidirectional porous hydroxyapatite body using ethanol bubbles in a viscous slurry. , J. Am. Ceram. Soc 91, 3125-3127.
- 486.Kawachi G, Misumi H, Fujimori H, Goto S, Ohtsuki C et al.Fabrication of porous blocks of calcium phosphate through hydrothermal processing under glycine coexistence. , J. Ceram. Soc. Jpn 2010, 559-563.
- 487.Sakamoto M, Nakasu M, Matsumoto T, Okihana H. (2007) Development of superporous hydroxyapatites and their examination with a culture of primary rat osteoblasts. , J. Biomed. Mater. Res. A 82-238.
- 488.Sakamoto M.Development and evaluation of superporous hydroxyapatite ceramics with triple pore structure as bone tissue scaffold. , J. Ceram. Soc. Jpn 2010, 753-757.
- 489.Deisinger U.Generating porous ceramic scaffolds: processing and properties. , Key Eng. Mater 2010, 155-179.
- 490.Ishikawa K, Tsuru K, T K Pham, Maruta M, Matsuya S. (2012) Fully-interconnected pore forming calcium phosphate cement. Key Eng. , Mater 493-494.
- 491.H J Yoon, U C Kim, J H Kim, Y H Koh, W Y Choi et al.Fabrication and characterization of highly porous calcium phosphate (CaP) ceramics by freezing foamed aqueous CaP suspensions. , J. Ceram. Soc. Jpn 2011, 573-576.
- 492.M K Ahn, K H Shin, Y W Moon, Y H Koh, W Y Choi et al.Highly porous biphasic calcium phosphate (BCP) ceramics with large interconnected pores by freezing vigorously foamed BCP suspensions under reduced pressure. , J. Am. Ceram. Soc 2011, 4154-4156.
- 493.Ji L, Jell G, Dong Y, J R, M.Template synthesis of ordered macroporous hydroxyapatite bioceramics. , Chem. Commun 2011, 9048-9050.
- 494.X Y Wang, Y C Han, S P Li. (2012) Preparation and characterization of calcium phosphate crystals by precursor thermolysis method. Key Eng. , Mater 493-494.
- 495.Schlosser M, H J Kleebe.Vapor transport sintering of porous calcium phosphate ceramics. , J. Am. Ceram. Soc 2012, 1581-1587.
- 496.Tanaka T, Yoshioka T, Ikoma T, Kuwayama T, Higaki T et al. (2013) Fabrication of three different types of porous carbonate-substituted apatite ceramics for artificial. 529-530.
- 497.Zheng W, Liu G, Yan C, Xiao Y, X G Miao.Strong and bioactive tri-calcium phosphate scaffolds with tube-like macropores. , J. Biomim. Biomater. Tissue Eng 2014, 65-75.
- 498.Tsuru K, Nikaido T, M L, Maruta M, Matsuya S et al. (2014) Synthesis of carbonate apatite foam using β-TCP foams as precursors. Key Eng. , Mater 587-52.
- 499.Z C Chen, X L Zhang, Zhou K, Cai H, C Q Liu.Novel fabrication of hierarchically porous hydroxyapatite scaffolds with refined porosity and suitable strength. , Adv. Appl. Ceram 2015, 183-187.
- 500.S K, Bhattacharyya S, Sarkar D. (2015) Fabrication of porous hydroxyapatite scaffold via polyethylene glycol-polyvinyl alcohol hydrogel state. , Mater. Res. Bull 64, 257-261.
- 501.Charbonnier B, Laurent C, Marchat D.Porous hydroxyapatite bioceramics produced by impregnation of 3D-printed wax mold: slurry feature optimization. , J. Eur. Ceram. Soc 2016, 4269-4279.
- 502.D M Roy, S K Linnehan. (1974) Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. , Nature 247-220.
- 503.Zhang X, K S Vecchio.Conversion of natural marine skeletons as scaffolds for bone tissue engineering. , Front. Mater. Sci 2013, 103-117.
- 504.Yang Y, Yao Q, Pu X, Hou Z, Zhang Q. (2011) Biphasic calcium phosphate macroporous scaffolds derived from oyster shells for bone tissue engineering. , Chem. Eng. J 173-837.
- 505.Thanh T N X, Maruta M, Tsuru K, Matsuya S, Ishikawa K. (2014) Three - dimensional porous carbonate apatite with sufficient mechanical strength as a bone substitute material. , Adv. Mater. Res 891-892.
- 506.A R Studart, U T Gonzenbach, Tervoort E, L J Gauckler. (2006) Processing routes to macroporous ceramics: a review. , J. Am. Ceram. Soc 89, 1771-1789.
- 507.Q L Loh, Choong C. (2013) Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. , Tissue Eng. B 19, 485-502.
- 508.Hing K, Annaz B, Saeed S, Revell P, Buckland T. (2005) Microporosity enhances bioactivity of synthetic bone graft substitutes. , J. Mater. Sci. Mater. Med 16, 467-475.
- 509.Wang Z, Sakakibara T, Sudo A, Kasai Y. (2013) Porosity of β-tricalcium phosphate affects the results of lumbar posterolateral fusion. , J. Spinal Disord. Tech 26-40.
- 510.Levengood Lan, S K Polak, S J Wheeler, M B, A J Clark et al. (2010) Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials. 31, 3552-3563.
- 511.Ruksudjarit A, Pengpat K, Rujijanagul G, Tunkasiri T. (2008) The fabrication of nanoporous hydroxyapatite ceramics. , Adv. Mater. Res 47-50.
- 512.Murugan R, Ramakrishna S, K P Rao. (2006) Nanoporous hydroxy-carbonate apatite scaffold made of natural. 60, 2844-2847.
- 513.Li Y, Tjandra W, K C Tam. (2008) Synthesis and characterization of nanoporous hydroxyapatite using cationic surfactants as templates. , Mater. Res. Bull 43, 2318-2326.
- 514.S El Asri, Laghzizil A, Saoiabi A, Alaoui A, K El Abassi et al. (2009) A novel process for the fabrication of nanoporous apatites from Moroccan phosphate rock. Colloid Surf. A. 350-73.
- 515.R A, Adnan R, M A Bakar, S M Masudi. (2011) Synthesis and characterisation of pure nanoporous hydroxyapatite. , J. Phys. Sci 22, 25-37.
- 517.Prokopiev O, Sevostianov I. (2006) Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature. , Mater. Sci. Eng. A 431-218.
- 518.Daculsi G, Jegoux F, Layrolle P. (2009) The micro macroporous biphasic calcium phosphate concept for bone reconstruction and tissue engineering. In: Advanced Biomaterials: Fundamentals, Processing. , Hoboken, NJ, USA 768.
- 519.Shipman P, Foster G, Schoeninger M. (1984) Burnt bones and teeth: an experimental study of color, morphology, crystal structure and shrinkage. , J. Archaeol. Sci 11, 307-325.
- 521.Wang H, Zhai L, Li Y, Shi T. (2008) Preparation of irregular mesoporous hydroxyapatite. , Mater. Res. Bull 43, 1607-1614.
- 522.Fan J, Lei J, Yu C, Tu B, Zhao D. (2007) Hard-templating synthesis of a novel rod-like nanoporous calcium phosphate bioceramics and their capacity as antibiotic carriers. , Mater. Chem. Phys 103-489.
- 523.Sopyan I, Mel M, Ramesh S, K A.Porous hydroxyapatite for artificial bone applications. , Sci. Technol. Adv. Mater 2007, 116-123.
- 524.Y H Hsu, I G Turner, A W Miles. (2007) Fabrication of porous bioceramics with porosity gradients similar to the bimodal structure of cortical and cancellous. 18, 2251-2256.
- 525.Abdurrahim T, Sopyan I.Recent progress on the development of porous bioactive calcium phosphate for biomedical applications. Recent Pat. , Biomed. Eng 2008, 213-229.
- 526.Munch E, Franco J, Deville S, Hunger P, Saiz E et al. (2008) Porous ceramic scaffolds with complex architectures. , JOM 60, 54-59.
- 527.Ohji T, Fukushima M. (2012) Macro-porous ceramics: processing and properties. , Int. Mater. Rev 57, 115-131.
- 528.A R Naqshbandi, Sopyan I, Gunawan.Development of porous calcium phosphate bioceramics for bone implant applications: a review. , Rec. Pat. Mater. Sci 2013, 238-252.
- 529.Yan X, Yu C, Zhou X, Tang J, Zhao D. (2004) Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. , Angew. Chem. Int. Ed. Engl 43, 5980-5984.
- 530.Izquierdo-Barba I, Ruiz-González L, J C Doadrio, J M González-Calbet, Vallet-Regí M. (2005) Tissue regeneration: a new property of mesoporous materials. Solid State Sci. 7, 983-989.
- 531.Cosijns A, Vervaet C, Luyten J, Mullens S, Siepmann F et al. (2007) Porous hydroxyapatite tablets as carriers for low-dosed drugs. , Eur. J. Pharm. Biopharm 67, 498-506.
- 532.Uchida A, Shinto Y, Araki N, Ono K. (1992) Slow release of anticancer drugs from porous calcium hydroxyapatite ceramic. , J. Orthop. Res 10, 440-445.
- 533.Shinto Y, Uchida A, Korkusuz F, Araki N, Ono K. (1992) Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. , J. Bone. Joint. Surg. Br 74, 600-604.
- 534.R B Martin, M W Chapman, N A Sharkey, S L Zissimos, Bay B et al. (1993) Bone ingrowth and mechanical properties of coralline hydroxyapatite 1 yr after implantation. Biomaterials. 14, 341-348.
- 535.G J Kazakia, E A Nauman, D M Ebenstein, B P Halloran, T M Keaveny. (2006) Effects of in vitro bone formation on the mechanical properties of a trabeculated hydroxyapatite bone substitute. , J. Biomed. Mater. Res. A 77-688.
- 536.K A Hing, S M Best, K E Tanner, Bonfield W, P A Revell. (2004) Mediation of bone ingrowth in porous hydroxyapatite bone graft substitutes. , J. Biomed. Mater. Res. A 68-187.
- 537.Vuola J, Taurio R, Goransson H, Asko-Seljavaara S. (1998) Compressive strength of calcium carbonate and hydroxyapatite implants after bone marrow induced osteogenesis. Biomaterials. 19, 223-227.
- 538.Doernberg M C von, B von Rechenberg, Bohner M, Grünenfelder S, Lenthe G H van et al. (2006) In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials. 27, 5186-5198.
- 539.Mygind T, Stiehler M, Baatrup A, Li H, Zou X et al. (2007) Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 28, 1036-1047.
- 540.M H, Afghani S, Franco J, Launey M, Marshall S et al. (2011) Lamellar spacing in cuboid hydroxyapatite scaffolds regulates bone formation by human bone marrow stromal cells. Tissue Eng. A. 17, 1615-1623.
- 541.Chan O, M J Coathup, Nesbitt A, C Y Ho, K A Hing et al. (2012) The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 8, 2788-2794.
- 542.R E Holmes. (1979) Bone regeneration within a coralline hydroxyapatite implant. , Plast. Reconstr. Surg 63, 626-633.
- 543.Tsuruga E, Takita H, Wakisaka Y, Kuboki Y. (1997) Pore size of porous hydoxyapatite as the cell-substratum controls BMP-induced osteogenesis. , J. Biochem 121-317.
- 544.R Z LeGeros, J P LeGeros. (2003) Calcium phosphate bioceramics: past, present, future. , Key Eng. Mater 240-242.
- 545.J R Woodard, A J Hilldore, S K Lan, C J Park, A W Morgan et al. (2007) The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 28, 45-54.
- 546.Michel J, Penna M, Kochen J, Cheung H. (2015) Recent advances in hydroxyapatite scaffolds containing mesenchymal stem cells. Stem Cells Int. 305217.
- 547.Mohammadian F, Eatemadi A.Drug loading and delivery using nanofibers scaffolds. , Artif. Cells Nanomed. Biotechnol 2017, 881-888.
- 548.Gautier H, Daculsi G, Merle C. (2001) Association of vancomycin and calcium phosphate by dynamic compaction: in vitro characterization and microbiological activity. Biomaterials. 22, 2481-2487.
- 549.Lebugle A, Rodrigues A, Bonnevialle P, J, Canal P et al. (2002) Study of implantable calcium phosphate systems for the slow release of methotrexate. Biomaterials. 23, 3517-3522.
- 550.Y L Liu, Enggist L, A F Kuffer, Buser D, E B Hunziker. (2007) The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials. 28, 2677-2686.
- 551.G H Nancollas, Tang R, R J Phipps, Henneman Z, Gulde S et al. (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 38, 617-627.
- 552.Errassif F, Menbaoui A, Autefage H, Benaziz L, Ouizat S et al. (2010) Adsorption on apatitic calcium phosphates: applications to drug delivery. In:. Advances in bioceramics , Hoboken, NJ, USA;, Ceramic Transactions 218, 159-174.
- 553.Kon M, Ishikawa K, Miyamoto Y, Asaoka K. (1995) Development of calcium phosphate based functional gradient bioceramics. Biomaterials. 16, 709-714.
- 554.L H Wong, Tio B, Miao X. (2002) Functionally graded tricalcium phosphate/fluoroapatite composites. , Mater. Sci. Eng. C 20, 111-115.
- 555.Tampieri A, Celotti G, Sprio S, Delcogliano A, Franzese S. (2001) Porosity-graded hydroxyapatite ceramics to replace natural bone. Biomaterials. 22, 1365-1370.
- 556.W, Zhao F, Luk K D K, Y J, Cheung K M C et al. (2003) Controllable porosity hydroxyapatite ceramics as spine cage: fabrication and properties. 14, 1039-1046.
- 557.Werner J, Linner-Krcmar B, Friess W, Greil P. (2002) Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials. 23, 4285-4294.
- 558.L M Rodriguez-, Ferreira J M F. (2004) Development of porous ceramic bodies for applications in tissue engineering and drug delivery systems. , Mater. Res. Bull 39, 83-91.
- 559.Watanabe T, Fukuhara T, Izui H, Fukase Y, Okano M. (2009) Properties of HAp/β-TCP functionally graded material by spark plasma sintering. , Trans. Jpn. Soc. Mech. Eng. A 75, 612-618.
- 560.Bai X, Sandukas S, M R Appleford, J L Ong, Rabiei A. (2009) Deposition and investigation of functionally graded calcium phosphate coatings on titanium. Acta Biomater. 5, 3563-3572.
- 561.Roy M, V K Balla, Bandyopadhyay A, Bose S. (2011) Compositionally graded hydroxyapatite/tricalcium phosphate coating on Ti by laser and induction plasma. Acta Biomater. 7, 866-873.
- 562.Tamura A, Asaoka T, Furukawa K, Ushida T, Tateishi T.Application of α-TCP/HAp functionally graded porous beads for bone regenerative scaffold. , Adv. Sci. Technol 2013, 63-69.
- 563.Gasik M, Keski-Honkola A, Bilotsky Y, Friman M.Development and optimisation of hydroxyapatite-β-TCP functionally gradated biomaterial. , J. Mech. Behav. Biomed. Mater 2014, 266-273.
- 564.Zhou C, Deng C, Chen X, Zhao X, Chen Y et al. (2015) Mechanical and biological properties of the micro-/nano-grain functionally graded hydroxyapatite bioceramics for bone tissue engineering. , J. Mech. Behav. Biomed. Mater 48, 1-11.
- 565.Marković S, M J Lukić, S D Škapin, Stojanović B, D. (2015) fabrication and characterization of nanostructured functionally graded HAp/BCP ceramics. , Ceram. Int 41, 2654-2667.
- 566.V A Dubok. (2000) Bioceramics – yesterday, today, tomorrow. , Powder Metall. Met. Ceram 39, 381-394.
- 568.R B Heimann. (2002) Materials science of crystalline bioceramics: a review of basic properties and applications. , CMU J 1, 23-46.
- 569.Ohtsuki C, Kamitakahara M, Miyazaki T. (2009) Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. , J. R. Soc. Interface 6, 349-360.
- 570.D C Greenspan. (1999) Bioactive ceramic implant materials. , Curr. Opin. Solid State Mater. Sci 4, 389-393.
- 571.T J Blokhuis, M F Termaat, Boer F C den, Patka P, F C Bakker et al. (2000) Properties of calcium phosphate ceramics in relation to their in vivo behavior. , J. Trauma 48, 179-189.
- 573.Seeley Z, Bandyopadhyay A, Bose S. (2008) Tricalcium phosphate based resorbable ceramics: influence of. , NaF and CaO addition. Mater. Sci. Eng. C 28, 11-17.
- 574.Leriche A J C. (2008) Synthesis of macroporous β-tricalcium phosphate with controlled porous architectural. , Ceram. Int 34, 1131-1137.
- 575.E K Cushnie, Y M Khan, C T Laurencin. (2008) Amorphous hydroxyapatite-sintered polymeric scaffolds for bone tissue regeneration: physical characterization studies. , J. Biomed. Mater. Res. A 84-54.
- 576.L, Thompson I. (2010) Twenty-first century challenges for biomaterials. , J. R. Soc. Interface 7, 379-391.
- 577.Nagase M, D G Baker, H R Schumacher. (1988) Prolonged inflammatory reactions induced by artificial ceramics in the rat pouch. 15, 1334-1338.
- 578.Rooney T, Berman S, A T Indersano. (1988) Evaluation of porous block hydroxylapatite for augmentation of alveolar ridges. , J. Oral Maxillofac. Surg 46, 15-18.
- 579.Prudhommeaux F, Schiltz C, Lioté F, Hina A, Champy R et al. (1996) Variation in the inflammatory properties of basic calcium phosphate crystals according to crystal type. Arthritis Rheum. 39, 1319-1326.
- 580.Ghanaati S, Barbeck M, Orth C, Willershausen I, B W Thimm et al. (2010) Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 6, 4476-4487.
- 581.Lin K, Yuan W, Wang L, Lu J, Chen L et al.Evaluation of host inflammatory responses of β-tricalcium phosphate bioceramics caused by calcium pyrophosphate impurity using a subcutaneous model. , J. Biomed. Mater. Res. B Appl. Biomater 2011, 350-358.
- 582.Velard F, Braux J, Amedee J, Laquerriere P. (2013) Inflammatory cell response to calcium phosphate biomaterial particles: an overview. Acta Biomater. 9, 4956-4963.
- 583.Rydén L, Molnar D, Esposito M, Johansson A, Suska F et al. (2013) Early inflammatory response in soft tissues induced by thin calcium phosphates. , J. Biomed. Mater. Res. A 101-2712.
- 584.Chatterjea A, Stok J van der, C B Danoux, Yuan H, Habibovic P et al. (2014) Inflammatory response and bone healing capacity of two porous calcium phosphate ceramics in a critical size cortical bone defects. , J. Biomed. Mater. Res. A 102-1399.
- 585.Friesenbichler J, Maurer-Ertl W, Sadoghi P, Pirker-Fruehauf U, Bodo K et al.Adverse reactions of artificial bone graft substitutes: lessons learned from using tricalcium phosphate geneX®. , Clin. Orthop. Relat. Res 2014, 976-982.
- 586.T Y Chang, S C Pan, Y H Huang, Y.Blindness after calcium hydroxylapatite injection at nose. , J. Plast. Reconstr. Aesthet. Surg 2014, 1755-1757.
- 587.P F Jacovella, C B Peiretti, Cunille D, Salzamendi M, S A. (2006) Long-lasting results with hydroxylapatite (Radiesse) facial filler. , Plast. Reconstr. Surg 118-15.
- 588.Ghanaati S, Barbeck M, Detsch R, Deisinger U, Hilbig U et al.The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. , Biomed. Mater 2012, 015005.
- 589.Draenert K, Draenert M, Erler M, Draenert A, Draenert Y. (2011) How bone forms in large cancellous defects: critical analysis based on experimental work and literature. Injury. 42, 47-55.
- 591.Yuan H, Kurashina K, Bruijn D J de, Li Y, K de Groot et al. (1999) A preliminary study of osteoinduction of two kinds of calcium phosphate bioceramics. Biomaterials. 20, 1799-1806.
- 592.H P Yuan, Bruijn J D de, Y B Li, J Q Feng, Z J Yang et al. (2001) Bone formation induced by calcium phosphate ceramics in soft tissue of dogs: a comparative study between porous α-TCP and. 12, 7-13.
- 593.Barrere F, Valk C M van der, R A Dalmeijer, Meijer G, Blitterswijk C A van et al. (2003) Osteogenecity of octacalcium phosphate coatings applied on porous titanium. , J. Biomed. Mater. Res. A 66-779.
- 594.Habibovic P, Valk C M van der, Blitterswijk C A van, K de Groot, Meijer G. (2004) Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. , J. Mater. Sci. Mater. Med 15, 373-380.
- 595.Ripamonti U, P W Richter, R W Nilen, Renton L. (2008) The induction of bone formation by smart biphasic hydroxyapatite tricalcium phosphate biomimetic matrices in the non human primate Papio ursinus. , J. Cell. Mol. Med 12, 2609-2621.
- 596.Cheng L, Ye F, Yang R, Lu X, Shi Y et al. (2010) Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 6, 1569-1574.
- 597.Yuan H, Fernandes H, Habibovic P, J de Boer, Barradas A M C et al. (2010) Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 107-13614.
- 598.J F Yao, X Y Li, A J Wang, Liang R, C Y Bao et al. (2011) Osteoinductive calcium phosphate ceramics for in vivo construction of tissue engineered bone in adipose tissue. , J. Clin. Rehabil. Tissue Eng. Res 15, 2109-2112.
- 599.A M Barradas, Yuan H, Stok J van der, B le Quang, Fernandes H et al. (2012) The influence of genetic factors on the osteoinductive potential of calcium phosphate ceramics in mice. Biomaterials. 33, 5696-5705.
- 600.Li B, Liao X, Zheng L, Zhu X, Wang Z et al. (2012) Effect of nanostructure on osteoinduction of porous biphasic calcium phosphate ceramics. Acta Biomater. 8, 3794-3804.
- 601.Cheng L, Shi Y, Ye F, Bu H. (2013) Osteoinduction of calcium phosphate biomaterials in small animals. , Mater. Sci. Eng. C 33, 1254-1260.
- 602.Song G, Habibovic P, Bao C, Hu J, Blitterswijk C A van et al. (2013) The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials. 34, 2167-2176.
- 603.He P, Sahoo S, K S Ng, Chen K, S L Toh et al. (2013) Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. , J. Biomed. Mater. Res. A 101-555.
- 604.N L Davison, A L Gamblin, Layrolle P, Yuan H, Bruijn J D de et al. (2014) Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials. 35, 5088-5097.
- 605.Huang Y, He J, Gan L, Liu X, Wu Y et al.Osteoconductivity and osteoinductivity of porous hydroxyapatite coatings deposited by liquid precursor plasma spraying: in vivo biological response study. , Biomed. Mater 2014, 065007.
- 606.Lü X, Wang J, Li B, Zhang Z, Zhao L. (2014) Gene expression profile study on osteoinductive effect of natural hydroxyapatite. , J. Biomed. Mater. Res. A 102-2833.
- 607.Wang J, Chen Y, Zhu X, Yuan T, Tan Y et al. (2014) Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. , J. Biomed. Mater. Res. A 102-4234.
- 608.Hongmin L, Wei Z, Xingrong Y, Jing W, Wenxin G et al. (2015) Osteoinductive nanohydroxyapatite bone substitute prepared via in situ hydrothermal transformation of cuttlefish. 103-816.
- 609.Wang L, Barbieri D, Zhou H, Bruijn J D de, Bao C et al. (2015) Effect of particle size on osteoinductive potential of microstructured biphasic calcium phosphate ceramic. , J. Biomed. Mater. Res. A 103-1919.
- 610.Cheng L, Wang T, Zhu J, Cai P. (2016) Osteoinduction of calcium phosphate ceramics in four kinds of animals for 1 year: dog, rabbit, rat, and mouse. Transplant. Proc 48, 1309-1314.
- 611.Habibovic P, Li J, Valk C M van der, Meijer G, Layrolle P et al. (2005) Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials. 26, 23-36.
- 612.Habibovic P, Yuan H, Valk C M van der, Meijer G, Blitterswijk C A van et al. (2005) 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 26, 3565-3575.
- 613.Habibovic P, T M Sees, Doel M A van den, Blitterswijk C A van, K de Groot. (2006) Osteoinduction by biomaterials – physicochemical and structural influences. , J. Biomed. Mater. Res. A 77-747.
- 614.A H Reddi. (2000) Morphogenesis and tissue engineering of bone and cartilage: Inductive signals, stem cells and biomimetic biomaterials. Tissue Eng. 6, 351-359.
- 615.Ripamonti U. (1991) The morphogenesis of bone in replicas of porous hydroxyapatite obtained by conversion of calcium carbonate exoskeletons of coral. , J. Bone Joint Surg. A 73, 692-703.
- 616.Kuboki Y, Takita H, Kobayashi D. (1998) BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. , J. Biomed. Mater. Res 39, 190-199.
- 617.Zhang J, Luo X, Barbieri D, Barradas A M C, Bruijn J D de et al. (2014) The size of surface microstructures as an osteogenic factor in calcium phosphate ceramics. Acta Biomater. 10, 3254-3263.
- 618.Zhang J, Barbieri D, Hoopen Ten, H de Bruijn, Blitterswijk J D van et al. (2015) Microporous calcium phosphate ceramics driving osteogenesis through surface architecture. , J. Biomed. Mater. Res. A 103-1188.
- 619.Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. (1992) Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. , Clin. Orthop. Relat. Res 275-280.
- 620.B D, Schwartz Z.Are calcium phosphate ceramics ‘smart’ biomaterials?. , Nat. Rev. Rheumatol 2011, 8-9.
- 621.Lu J, Descamps M, Dejou J, Koubi G, Hardouin P et al. (2002) The biodegradation mechanism of calcium phosphate biomaterials in bone. , J. Biomed. Mater. Res. Appl. Biomater 63, 408-412.
- 622.Wang H, J K Lee, Moursi A, J. (2003) Ca/P ratio effects on the degradation of hydroxyapatite in vitro. , J. Biomed. Mater. Res. A 67-599.
- 623.S V Dorozhkin. (1999) Inorganic chemistry of the dissolution phenomenon, the dissolution mechanism of calcium apatites at the atomic (ionic) level. , Comment Inorg. Chem 20, 285-299.
- 624.S V Dorozhkin. (2012) Dissolution mechanism of calcium apatites in acids: a review of literature. 2, 1-17.
- 625.Sakai S, Anada T, Tsuchiya K, Yamazaki H, H C Margolis et al. (2016) Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. 35, 216-224.
- 626.Wenisch S, J P Stahl, Horas U, Heiss C, Kilian O et al. (2003) In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: fine structural microscopy. , J. Biomed. Mater. Res. A 67-713.
- 627.Riihonen R, Nielsen S, H K Väänänen, Laitala-Leinonen T, T H Kwon. (2010) Degradation of hydroxyapatite in vivo and in vitro requires osteoclastic sodium-bicarbonate co-transporter NBCn1. Matrix Biol. 29, 287-294.
- 629.Matsunaga A, Takami M, Irié T, Mishima K, Inagaki K et al. (2015) Microscopic study on resorption of β-tricalcium phosphate materials by osteoclasts. Cytotechnology. 67, 727-732.
- 630.Narducci P, Nicolin V. (2009) Differentiation of activated monocytes into osteoclast-like cells on a hydroxyapatite substrate: an in vitro study. , Ann. Anat 191-349.
- 631.V M Wu, Uskoković V. (2016) Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro?. , Biochim. Biophys. Acta 1860, 2157-2168.
- 632.Tamimi F, Torres J, Bassett D, Barralet J, E L Cabarcos. (2010) Resorption of monetite granules in alveolar bone defects in human patients. Biomaterials. 31, 2762-2769.
- 633.Sheikh Z, M N Abdallah, A, Misbahuddin S, Rashid H et al. (2015) Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials. 8, 7913-7925.
- 634.Raynaud S, Champion E, J P Lafon, Bernache-Assollant D. (2002) Calcium phosphate apatites with variable Ca/P atomic ratio. III. Mechanical properties and degradation in solution of hot pressed ceramics. Biomaterials. 23, 1081-1089.
- 635.Barrère F, Valk C M van der, Dalmeijer R A J, Blitterswijk C A van, K de Groot et al. (2003) In vitro and in vivo degradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants. , J. Biomed. Mater. Res. A 64-378.
- 636.R M Souto, M, R L. (2003) Degradation characteristics of hydroxyapatite coatings on orthopaedic TiAlV in simulated physiological media investigated by electrochemical impedance spectroscopy. Biomaterials. 24, 4213-4221.
- 637.J G Dellinger, A M Wojtowicz, R D Jamison. (2006) Effects of degradation and porosity on the load bearing properties of model hydroxyapatite bone scaffolds. , J. Biomed. Mater. Res. A 77-563.
- 638.Okuda T, Ioku K, Yonezawa I, Minagi H, Kawachi G et al. (2007) The effect of the microstructure of β-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials. 28, 2612-2621.
- 639.Okada M, Furukawa K, Serizawa T, Yanagisawa Y, Tanaka H et al. (2009) Interfacial interactions between calcined hydroxyapatite nanocrystals and substrates. Langmuir. 25, 6300-6306.
- 640.P D Callis, Donaldson K, J F McCord.Early cellular responses to calcium phosphate ceramics. , Clin. Mater 1988, 183-190.
- 641.Okumura M, Ohgushi H, Tamai S. (1990) Bonding osteogenesis in coralline hydroxyapatite combined with bone marrow cells. Biomaterials. 12, 28-37.
- 642.E A Holtgrave, Donath K. (1995) Response of odontoblast-like cells to hydroxyapatite ceramic granules. Biomaterials. 16, 155-159.
- 643.Doi Y, Iwanaga H, Shibutani T, Moriwaki Y, Iwayama Y. (1999) Osteoclastic responses to various calcium phosphates in cell cultures. , J. Biomed. Mater. Res 47, 424-433.
- 644.Guo X, J E Gough, Xiao P, Liu J, Shen Z. (2007) Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. , J. Biomed. Mater. Res. A 82-1022.
- 645.Wang Y, Zhang S, Zeng X, L, Weng W et al. (2007) Osteoblastic cell response on fluoridated hydroxyapatite coatings. Acta Biomater. 3, 191-197.
- 646.W J Bae, S W Chang, S I Lee, K Y, K S Bae et al.Human periodontal ligament cell response to a newly developed calcium phosphate-based root canal sealer. , J. Endod 2010, 1658-1663.
- 647.Li J, Song Y, Zhang S, Zhao C, Zhang F et al. (2010) In vitro responses of human bone marrow stromal cells to a fluoridated hydroxyapatite coated biodegradable Mg-Zn alloy. Biomaterials. 31, 5782-5788.
- 648.Zhao X, B C Heng, Xiong S, Guo J, T et al. (2011) In vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areas. Nanotoxicology. 5, 182-194.
- 649.Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H et al.In vitro-osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. , J. Biomater. Appl 2011, 359-380.
- 650.Kanayama K, Sriarj W, Shimokawa H, Ohya K, Doi Y et al.Osteoclast and osteoblast activities on carbonate apatite plates in cell cultures. , J. Biomater. Appl 2011, 435-449.
- 651.Liu X, Zhao M, Lu J, Ma J, Wei J et al.Cell responses to two kinds of nanohydroxyapatite with different sizes and crystallinities. , Int. J. Nanomed 2012, 1239-1250.
- 652.Marchi J, Ribeiro C, de Almeida Bressiant, A H Marquesd, M. (2013) Cell response of calcium phosphate based ceramics, a bone substitute material. , Mater. Res 16, 703-712.
- 653.R A Perez, T H Kim, Kim M, J H, M P Ginebra et al. (2013) Calcium phosphate cements loaded with basic fibroblast growth factor: delivery and in vitro cell response. , J. Biomed. Mater. Res. A 101-923.
- 654.Yin P, F, Lei T, X H Zhong, X C Jian. (2014) Osteoblastic cell response on biphasic fluorhydroxyapatite/strontium-substituted hydroxyapatite coatings. , J. Biomed. Mater. Res. A 102-621.
- 655.S E Lobo, Glickman R, Silva da, W N Arinzeh, T L Kerkis et al. (2015) Response of stem cells from different origins to biphasic calcium phosphate bioceramics. Cell Tiss. Res. 361-477.
- 656.Suzuki T, Ohashi R, Yokogawa Y, Nishizawa K, Nagata F et al. (1999) Initial anchoring and proliferation of fibroblast L-929 cells on unstable surface of calcium phosphate ceramics. , J. Biosci. Bioeng 87, 320-327.
- 657.T L Arinzeh, Tran T, McAlary J, Daculsi G. (2005) A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 26, 3631-3638.
- 658.Oh S, Oh N, Appleford M, J L Ong.Bioceramics for tissue engineering applications – a review. , Am. J. Biochem. Biotechnol 2006, 49-56.
- 659.Appleford M, Oh S, J A Cole, D L Carnes, Lee M et al. (2007) Effects of trabecular calcium phosphate scaffolds on stress signaling in osteoblast precursor cells. Biomaterials. 28, 2747-2753.
- 660.Gamie Z, G T Tran, Vyzas G, Korres N, Heliotis M et al.Stem cells combined with bone graft substitutes in skeletal tissue engineering. , Expert Opin. Biol. Ther 2012, 713-729.
- 661.Manfrini M, C di Bona, Canella A, Lucarelli E, Pellati A et al. (2013) Mesenchymal stem cells from patients to assay bone graft substitutes. , J. Cell. Physiol 228-1229.
- 662.R E Unger, Sartoris A, Peters K, Motta A, Migliaresi C et al. (2007) Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary like structures on three-dimensional porous biomaterials. Biomaterials. 28, 3965-3976.
- 663.N M, Dasmawati M, S M Azman, N S Omar, Othman R.Biocompatibility of in house β-tricalcium phosphate ceramics with normal human osteoblast cell. , J. Eng. Sci. Technol 2012, 169-176.
- 664.Tan F, O’Neill F, Naciri M, Dowling D, Al-Rubeai M. (2012) Cellular and transcriptomic analysis of human mesenchymal stem cell response to plasma-activated hydroxyapatite coating. Acta Biomater. 8, 1627-1638.
- 665.Li B, Liao X, Zheng L, He H, Wang H et al. (2012) Preparation and cellular response of porous A-type carbonated hydroxyapatite nanoceramics. , Mater. Sci. Eng. C 32, 929-936.
- 666.Teixeira S, M H Fernandes, M P Ferraz, F J Monteiro. (2010) Proliferation and mineralization of bone marrow cells cultured on macroporous hydroxyapatite scaffolds functionalized with collagen type I for bone tissue regeneration. , J. Biomed. Mater. Res. A 95-1.
- 667.Yan-Zhong Z, Yan-Yan H, Jun Z, Shai-Hong Z, Zhi-You L et al.Characteristics of functionalized nano-hydroxyapatite and internalization by human epithelial cell. , Nanoscale Res. Lett 600(8), pages)..
- 668.Borcard F, Staedler D, Comas H, F K Juillerat, P N Sturzenegger et al.Chemical functionalization of bioceramics to enhance endothelial cells adhesion for tissue engineering. , J. Med. Chem 2012, 7988-7997.
- 669.Treccani L, T Y Klein, Meder F, Pardun K, Rezwan K. (2013) Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 9, 7115-7150.
- 670.Russo L, Taraballi F, Lupo C, Poveda A, Jiménez-Barbero J et al. (2014) Carbonate hydroxyapatite functionalization: a comparative study towards (bio)molecules fixation. Interface Focus. 4, 20130040.
- 671.Zhuang Z, Yoshimura H, Aizawa M. (2013) Synthesis and ultrastructure of plate-like apatite single crystals as a model for tooth enamel. , Mater. Sci. Eng. C 33, 2534-2540.
- 672.Zhuang Z, T J Fujimi, Nakamura M, Konishi T, Yoshimura H et al. (2013) Development of a,b-plane-oriented hydroxyapatite ceramics as models for living bones and their cell adhesion behavior. Acta Biomater. 9, 6732-6740.
- 673.Aizawa M, Matsuura T, Zhuang Z.Syntheses of single-crystal apatite particles with preferred orientation to the a- and c-axes as models of hard tissue and their applications. , Biol. Pharm. Bull 2013, 1654-1661.
- 674.Lin K, Wu C, Chang J. (2014) Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 10, 4071-4102.
- 675.Chen W, Long T, Y J Guo, Z A, Y P Guo. (2014) Hydrothermal synthesis of hydroxyapatite coatings with oriented nanorod arrays. RSC Adv. 4, 185-191.
- 676.J, Tian B, Tang S, Q F Ke, C Q Zhang et al. (2015) Hydroxyapatite coatings with oriented nanoplate arrays: synthesis, formation mechanism and cytocompatibility. , J. Mater. Chem. B 3, 1655-1666.
- 679.Barrère F, T A Mahmood, K de Groot, Blitterswijk C A van. (2008) Advanced biomaterials for skeletal tissue regeneration: instructive and smart functions. , Mater. Sci. Eng. R 59, 38-71.
- 680.Liu H, T J Webster. (2007) Nanomedicine for implants: a review of studies and necessary experimental tools. Biomaterials. 28, 354-369.
- 681.Wang C, Duan Y, Markovic B, Barbara J, C R Howlett et al. (2004) Proliferation and bone-related gene expression of osteoblasts grown on hydroxyapatite ceramics sintered at different temperature. Biomaterials. 25, 2949-2956.
- 682.Samavedi S, A R Whittington, A S Goldstein. (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 9, 8037-8045.
- 683.Matsumoto T, Okazaki M, Nakahira A, Sasaki J, Egusa H et al. (2007) Modification of apatite materials for bone tissue engineering and drug delivery carriers. , Curr. Med. Chem 14, 2726-2733.
- 684.Y C Chai, Carlier A, Bolander J, S J Roberts, Geris L et al. (2012) Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 8, 3876-3887.
- 685.Denry I, L T Kuhn. (2016) Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. , Dent. Mater 32, 43-53.
- 686.Vallet-Regí M, Arcos D. (2005) Silicon substituted hydroxyapatites. A method to upgrade calcium phosphate based implants. , J. Mater. Chem 15, 1509-1516.
- 687.Gbureck U, Thull R, J E Barralet. (2005) Alkali ion substituted calcium phosphate cement formation from mechanically activated reactants. , J. Mater. Sci. Mater. Med 16, 423-427.
- 688.Boanini E, Gazzano M, Bigi A. (2010) Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 6, 1882-1894.
- 689.J H Shepherd, D V Shepherd, S M Best. (2012) Substituted hydroxyapatites for bone repair. , J. Mater. Sci. Mater. Med 23, 2335-2347.
- 690.Šupová M. (2015) Substituted hydroxyapatites for biomedical applications: a review. , Ceram. Int 41, 9203-9231.
- 691.Traykova T, Aparicio C, M P Ginebra, J A Planell. (2006) Bioceramics as nanomaterials. Nanomedicine. 1, 91-106.
- 692.S J Kalita, Bhardwaj A, H A Bhatt. (2007) Nanocrystalline calcium phosphate ceramics in biomedical engineering. , Mater. Sci. Eng. C 27, 441-449.
- 693.S V Dorozhkin.Nanodimensional and nanocrystalline calcium orthophosphates. , Int. J. Chem. Mater. Sci 2013, 105-174.
- 694.Šupová M. (2014) Isolation and preparation of nanoscale bioapatites from natural sources: a review. , J. Nanosci. Nanotechnol 14, 546-563.
- 695.Zhao J, Liu Y, W B Sun, Zhang H.Amorphous calcium phosphate and its application in dentistry. , Chem. Cent. J 40(7), pages)..
- 696.S V Dorozhkin. () Amorphous calcium orthophosphates: nature, chemistry and biomedical applications. , Int. J. Mater. Chem 2012.
- 697.Liu B, D X Lun.Current application of β-tricalcium phosphate composites in orthopaedics. , Orthop. Surg 2012, 139-144.
- 698.Venkatesan J, S K Kim. (2014) Nano-hydroxyapatite composite biomaterials for bone tissue engineering – a review. , J. Biomed. Nanotechnol 10, 3124-3140.
- 699.Wu Y, L, Du J, K L Choy, Guo J. (2004) Preparation of hydroxyapatite fibers by electrospinning technique. , J. Am. Ceram. Soc 87, 1988-1991.
- 700.S R Ramanan, Venkatesh R. (2004) A study of hydroxyapatite fibers prepared via sol-gel route. , Mater. Lett 58, 3320-3323.
- 701.Aizawa M, A E Porter, S M Best, Bonfield W. (2005) Ultrastructural observation of single-crystal apatite fibres. Biomaterials. 26, 3427-3433.
- 702.Y M Park, S C Ryu, S Y Yoon, Stevens R, H C Park. (2008) Preparation of whisker-shaped hydroxyapatite / β-tricalcium phosphate composite. , Mater. Chem. Phys 109-440.
- 703.Aizawa M, Ueno H, Itatani K, Okada I. (2006) Syntheses of calcium-deficient apatite fibres by a homogeneous precipitation method and their characterizations. , J. Eur. Ceram. Soc 26, 501-507.
- 704.D S Seo, J K Lee. (2008) Synthesis of hydroxyapatite whiskers through dissolution-reprecipitation process using EDTA. , J. Cryst. Growth 310-2162.
- 705.A C Tas. (2007) Formation of calcium phosphate whiskers in hydrogen peroxide (H2O2) solutions at 90°C. , J. Am. Ceram. Soc 90, 2358-2362.
- 706.I S Neira, Guitian F, Taniguchi T, Watanabe T, Yoshimura M. (2008) Hydrothermal synthesis of hydroxyapatite whiskers with sharp faceted hexagonal morphology. , J. Mater. Sci 43, 2171-2178.
- 707.H Y Yang, S F Yang, X P Chi, Evans J R G, Thompson I et al. (2008) Sintering behaviour of calcium phosphate filaments for use as hard tissue scaffolds. , J. Eur. Ceram. Soc 28, 159-167.
- 708.Junginger M, Kübel C, F H Schacher, Müller A H E, Taubert A. (2013) Crystal structure and chemical composition of biomimetic calcium phosphate nanofibers. RSC Adv. 3, 11301-11308.
- 709.Y S Cui, T, X P Wu, Q H Chen.Preparation and characterization of hydroxyapatite whiskers. , Appl. Mech. Mater 2013, 21-24.
- 710.J H Lee, Y J Kim. (2014) Hydroxyapatite nanofibers fabricated through electrospinning and sol-gel process. , Ceram. Int 40, 3361-3369.
- 711.Zhang H, Zhu Q. (2009) Synthesis of nanospherical and ultralong fibrous hydroxyapatite and reinforcement of biodegradable chitosan/hydroxyapatite composite. , Modern Phys. Lett. B 23, 3967-3976.
- 712.Wijesinghe W P S L, Mantilaka M M M G P G, Premalal E V A, Herath H M T U, Mahalingam S et al. (2014) Facile synthesis of both needle-like and spherical hydroxyapatite nanoparticles: effect of synthetic temperature and calcination on morphology, crystallite size and crystallinity. , Mater. Sci. Eng. C 42, 83-90.
- 713.C, C, M A Barbosa. (2006) Preparation and characterisation of calcium-phosphate porous microspheres with a uniform size for biomedical applications. , J. Mater. Sci. Mater. Med 17, 455-463.
- 714.Kimura I, Honma T, R E. (2007) Preparation of hydroxyapatite microspheres by interfacial reaction in a multiple emulsion. , J. Ceram. Soc. Jpn 115-888.
- 715.W Y Zhou, Wang M, W L Cheung, B C Guo, D M Jia. (2008) Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. , J. Mater. Sci. Mater. Med 19, 103-110.
- 716.J H Lim, J H Park, E K Park, H J Kim, I K Park et al. (2008) Fully interconnected globular porous biphasic calcium phosphate ceramic scaffold facilitates osteogenic repair. Key Eng. , Mater 361-363.
- 717.Kawai T, Sekikawa H, Unuma H. (2009) Preparation of hollow hydroxyapatite microspheres utilizing poly(divinylbenzene) as a template. , J. Ceram. Soc. Jpn 117-340.
- 718.Descamps M, J C Hornez, Leriche A. (2009) Manufacture of hydroxyapatite beads for medical applications. , J. Eur. Ceram. Soc 29, 369-375.
- 719.J S Cho, D S Jung, J M Han, Y C Kang.Spherical shape hydroxyapatite powders prepared by flame spray pyrolysis. , J. Ceram. Process. Res 2008, 348-352.
- 720.Yao A, Ai F, Liu X, Wang D, Huang W et al.Preparation of hollow hydroxyapatite microspheres by the conversion of borate glass at near room temperature. , Mater. Res. Bull 2010, 25-28.
- 721.J S Cho, Y N Ko, H Y Koo, Y C Kang.Synthesis of nano-sized biphasic calcium phosphate ceramics with spherical shape by flame spray pyrolysis. , J. Mater. Sci. Mater. Med 2010, 1143-1149.
- 722.Ye F, Guo H, Zhang H, He X. (2010) Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 6, 2212-2218.
- 723.He W, Tao J, Pan H, Xu R, Tang R.A size-controlled synthesis of hollow apatite nanospheres at water-oil interfaces. , Chem. Lett 2010, 674-675.
- 724.Itatani K, Tsugawa T, Umeda T, Musha Y, I J Davies et al.Preparation of submicrometer-sized porous spherical hydroxyapatite agglomerates by ultrasonic spray pyrolysis technique. , J. Ceram. Soc. Jpn 2010, 462-466.
- 725.Xiao W, Fu H, M N Rahaman, Liu Y, B S. (2013) Hollow hydroxyapatite microspheres: a novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater. 9, 8374-8383.
- 726.Bohner M, Tadier S, N van Garderen, A de Gasparo, Döbelin N et al. (2013) Synthesis of spherical calcium phosphate particles for dental and orthopedic applications. Biomatter. 3, 25103.
- 727.M N Rahaman, Fu H, Xiao W, Liu Y.Bioactive ceramic implants composed of hollow hydroxyapatite microspheres for bone regeneration. , Ceram. Eng. Sci. Proc 2014, 67-76.
- 728.Ito N, Kamitakahara M, Ioku K.Preparation and evaluation of spherical porous granules of octacalcium phosphate/hydroxyapatite as drug carriers in bone cancer treatment. , Mater. Lett 2014, 94-96.
- 729.Li Z, Wen T, Su Y, Wei X, He C et al. (2014) Hollow hydroxyapatite spheres fabrication with three-dimensional hydrogel template. CrystEngComm. 16, 4202-4209.
- 730.Feng J, Chong M, Chan J, Z Y, S H Teoh et al.characterization and in-vitro evaluation of apatite-based microbeads for bone implant science. , Ceram. Transact 2014, 179-190.
- 731.Kovach I, Kosmella S, Prietzel C, Bagdahn C, Koetz J. (2015) Nano-porous calcium phosphate balls. Colloid Surf. B. 132-246.
- 732.Kamitakahara M, Murakami S, Takahashi H, Watanabe N, Ioku K.Formation of hydroxyapatite microtubes assisted with anatase under hydrothermal conditions. , Chem. Lett 2010, 854-855.
- 733.Chandanshive B, Dyondi D, V R Ajgaonkar, Banerjee R, Khushalani D. (2010) Biocompatible calcium phosphate based tubes. , J. Mater. Chem 20, 6923-6928.
- 734.Kamitakahara M, Takahashi H, Ioku K.Tubular hydroxyapatite formation through a hydrothermal process from α-tricalcium phosphate with anatase. , J. Mater. Sci 2012, 4194-4199.
- 735.C B Ustundag, Kaya F, Kamitakahara M, Kaya C, Ioku K.Production of tubular porous hydroxyapatite using electrophoretic deposition. , J. Ceram. Soc. Jpn 2012, 569-573.
- 736.Li C, Ge X, Li G, Lu H, Ding R. (2014) In situ hydrothermal crystallization of hexagonal hydroxyapatite tubes from yttrium ion-doped hydroxyapatite by the Kirkendall effect. , Mater. Sci. Eng. C 45, 191-195.
- 737.Nonoyama T, Kinoshita T, Higuchi M, Nagata K, Tanaka M et al.Arrangement techniques of proteins and cells using amorphous calcium phosphate nanofiber scaffolds. , Appl. Surf. Sci 2012, 8-12.
- 738.Sohier J, Daculsi G, Sourice S, K de Groot, Layrolle P. (2010) Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. , J. Biomed. Mater. Res. A 92-1105.
- 739.Stähli C, Bohner M, Bashoor-Zadeh M, Doebelin N, Baroud G. (2010) Aqueous impregnation of porous β-tricalcium phosphate scaffolds. Acta Biomater. 6, 2760-2772.
- 740.Lin K, Chen L, Qu H, Lu J, Chang J.Improvement of mechanical properties of macroporous β-tricalcium phosphate bioceramic scaffolds with uniform and interconnected pore structures. , Ceram. Int 2011, 2397-2403.
- 741.Wójtowicz J, Leszczyńska J, Chróścicka A, Ślósarczyk A, Paszkiewicz Z et al.Comparative in vitro study of calcium phosphate ceramics for their potency as scaffolds for tissue engineering. , Bio-Med. Mater. Eng 2014, 1609-1623.
- 742.J L Simon, Michna S, J A Lewis, E D Rekow, V P Thompson et al. (2007) In vivo bone response to 3D periodic hydroxyapatite scaffolds assembled by direct ink writing. , J. Biomed. Mater. Res. A 83-747.
- 743.Yoshikawa H, Myoui A. (2005) Bone tissue engineering with porous hydroxyapatite ceramics. , J. Artif. Organs 8, 131-136.
- 744.S H Min, H, H Y Park, I M Park, H C Park et al. (2006) Preparation of porous hydroxyapatite scaffolds for bone tissue engineering. , Mater. Sci. Forum 510-511.
- 745.Deville S, Saiz E, R K Nalla, A P Tomsia. (2006) Strong biomimetic hydroxyapatite scaffolds. , Adv. Sci. Technol 49, 148-152.
- 746.C T Buckley, K U O’.Fabrication and characterization of a porous multidomain hydroxyapatite scaffold for bone tissue engineering investigations. , J. Biomed. Mater. Res. B Appl. Biomater 2010, 459-467.
- 747.Ramay H R R, Zhang M. (2004) Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials. 25, 5171-5180.
- 748.Chen G, Li W, Zhao B, Sun K. (2009) A novel biphasic bone scaffold: β-calcium phosphate and amorphous calcium polyphosphate. , J. Am. Ceram. Soc 92, 945-948.
- 749.Guo D, Xu K, Han Y. (2009) The in situ synthesis of biphasic calcium phosphate scaffolds with controllable compositions, structures, and adjustable properties. , J. Biomed. Mater. Res. A 88-43.
- 750.Sarin P, S J Lee, Z D Apostolov, W M Kriven.Porous biphasic calcium phosphate scaffolds from cuttlefish bone. , J. Am. Ceram. Soc 2011, 2362-2370.
- 751.D H Kim, K L, H, T W Kim, H C Park et al.In vitro biodegradable and mechanical performance of biphasic calcium phosphate porous scaffolds with unidirectional macro-pore structure. , Ceram. Int 2014, 8293-8300.
- 752.C F Marques, F H Perera, Marote A, Ferreira S, S I Vieira et al.Biphasic calcium phosphate scaffolds fabricated by direct write assembly: mechanical, anti-microbial and osteoblastic properties. , J. Eur. Ceram. Soc 2017, 359-368.
- 753.Furuichi K, Oaki Y, Ichimiya H, Komotori J, Imai H.Preparation of hierarchically organized calcium phosphate-organic polymer composites by calcification of hydrogel. , Sci. Technol. Adv. Mater 2006, 219-225.
- 754.Wei J, Jia J, Wu F, Wei S, Zhou H et al. (2010) Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials. 31, 1260-1269.
- 755.Gbureck U, Grolms O, J E Barralet, L M Grover, Thull R. (2003) Mechanical activation and cement formation of β-tricalcium phosphate. Biomaterials. 24, 4123-4131.
- 756.Gbureck U, J E Barralet, Hofmann M, Thull R. (2004) Mechanical activation of tetracalcium phosphate. , J. Am. Ceram. Soc 87, 311-313.
- 757.Bohner M, Luginbühl R, Reber C, Doebelin N, Baroud G et al. (2009) A physical approach to modify the hydraulic reactivity of α-tricalcium phosphate powder. Acta Biomater. 5, 3524-3535.
- 758.Hagio T, Tanase T, Akiyama J, Iwai K, Asai S. (2008) Formation and biological affinity evaluation of crystallographically aligned hydroxyapatite. , J. Ceram. Soc. Jpn 116-79.
- 759.Quarto R, Mastrogiacomo M, Cancedda R, S M Kutepov, Mukhachev V et al. (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. 344-385.
- 760.C A Vacanti, L J Bonassar, M P Vacanti, Shufflebarger J. (2001) Replacement of an avulsed phalanx with tissue-engineered. 344-1511.
- 761.Morishita T, Honoki K, Ohgushi H, Kotobuki N, Matsushima A et al. (2006) Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients’ mesenchymal stem cells. , Artif. Organs 30, 115-118.
- 762.J O Eniwumide, Yuan H, S H Cartmell, G J Meijer, Bruijn J D de. (2007) Ectopic bone formation in bone marrow stem cell seeded calcium phosphate scaffolds as compared to autograft and (cell seeded) allograft. Eur. Cell Mater. 14, 30-39.
- 763.Zhuang Y, Gan Y, Shi D, Zhao J, Tang T et al.A novel cytotherapy device for rapid screening, enriching and combining mesenchymal stem cells into a biomaterial for promoting bone regeneration. , Sci. Rep 2017, 15463.
- 764.Zuolin J, Hong Q, Jiali T. (2010) Dental follicle cells combined with beta-tricalcium phosphate ceramic: a novel available therapeutic strategy to restore periodontal defects. Med. Hypotheses. 75, 669-670.
- 765.Ge S, Zhao N, Wang L, Yu M, Liu H et al.Bone repair by periodontal ligament stem cell-seeded nanohydroxyapatite-chitosan scaffold. , Int. J. Nanomed 2012, 5405-5414.
- 766.Franch J, Díaz-Bertrana C, Lafuente P, Fontecha P, Durall I. (2006) Beta-tricalcium phosphate as a synthetic cancellous bone graft in veterinary orthopaedics: a retrospective study of 13 clinical cases. , Vet. Comp. Orthop. Traumatol 19, 196-204.
Cited by (21)
- 1.Baudín Carmen, Pena Pilar, 2023, Review: Tailored microstructures in the system tricalcium phosphate-wollastonite-diopside for bone regeneration scaffolds, Open Ceramics, 16(), 100483, 10.1016/j.oceram.2023.100483
- 2.Daskalova Albena, Sezanova Kostadinka, Angelova Liliya, Paunova-Krasteva Tsvetelina, Gergulova Rumiana, et al, 2023, Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity, Materials, 16(20), 6626, 10.3390/ma16206626
- 3.Lakrat Mohammed, Mejdoubi El Miloud, Ozdemir Fatma, Santos Catarina, 2022, Effect of sodium silicate concentration on the physico-chemical properties of dual-setting bone-like apatite cements, Materials Today Communications, 31(), 103421, 10.1016/j.mtcomm.2022.103421
- 4.Bulina Natalia V., Makarova Svetlana V., Baev Sergey G., Matvienko Alexander A., Gerasimov Konstantin B., et al, 2021, A Study of Thermal Stability of Hydroxyapatite, Minerals, 11(12), 1310, 10.3390/min11121310
- 5.Maslova L. Yu., Krut’ko V. K., Musskaya O. N., Safronova T. V., Kulak A. I., 2023, Formation of Biomimetic Apatite on Calcium Phosphate Foam Ceramic in a Concentrated Model Solution, Inorganic Materials: Applied Research, 14(2), 392, 10.1134/S2075113323020314
- 6.Ahmed Lana Omar, Bulut Niyazi, Kebiroglu Hanifi, Alkhedher Mohammad, Ates Tankut, et al, 2022, Effects of Yttrium Doping on Erbium-Based Hydroxyapatites: Theoretical and Experimental Study, Materials, 15(20), 7211, 10.3390/ma15207211
- 7.Bystrom Joseph Lazraq, Pujari-Palmer Michael, 2019, Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro, Journal of Functional Biomaterials, 10(4), 54, 10.3390/jfb10040054
- 8.del Valle García Adrián, Hautcoeur Dominique, Leriche Anne, Cambier Francis, Baudín Carmen, 2020, Microstructural design of ceramics for bone regeneration, Journal of the European Ceramic Society, 40(7), 2555, 10.1016/j.jeurceramsoc.2019.10.039
- 9.Nahanmoghadam Amir, Asemani Maryam, Goodarzi Vahabodin, Ebrahimi‐Barough Somayeh, 2021, Design and fabrication of bone tissue scaffolds based on PCL/PHBV containing hydroxyapatite nanoparticles: dual‐leaching technique, Journal of Biomedical Materials Research Part A, 109(6), 981, 10.1002/jbm.a.37087
- 10.Agnes Celine J., Karoichan Antoine, Tabrizian Maryam, 2023, The Diamond Concept Enigma: Recent Trends of Its Implementation in Cross-linked Chitosan-Based Scaffolds for Bone Tissue Engineering, ACS Applied Bio Materials, 6(7), 2515, 10.1021/acsabm.3c00108
- 11.M.N. Muhammad Syazwan, M.N. Ahmad-Fauzi, Balestri W., Reinwald Y., B.I. Yanny Marliana, 2021, Effectiveness of various sintering aids on the densification and in vitro properties of carbonated hydroxyapatite porous scaffolds produced by foam replication technique, Materials Today Communications, 27(), 102395, 10.1016/j.mtcomm.2021.102395
- 12.Miri Zahra, Haugen Håvard Jostein, Loca Dagnija, Rossi Filippo, Perale Giuseppe, et al, 2024, Review on the strategies to improve the mechanical strength of highly porous bone bioceramic scaffolds, Journal of the European Ceramic Society, 44(1), 23, 10.1016/j.jeurceramsoc.2023.09.003
- 13.Bulina N. V., Titkov A. I., Isaev D. D., Makarova S. V., Baev S. G., et al, 2021, Selective laser melting of Zn-Si-substituted hydroxyapatite, Russian Chemical Bulletin, 70(9), 1682, 10.1007/s11172-021-3270-8
- 14.Ferro Alberto C., Seixas Tomás, Guedes Mafalda, 2024, Reaction path in the mechanosynthesis of calcium phosphates using a biogenic calcium source, Ceramics International, 50(1), 282, 10.1016/j.ceramint.2023.10.102
- 15.Ribeiro Suzana Barreto Noronha, da Veiga Junior Valdir Florêncio, de Campos José Brant, dos Santos Jheison Lopes, Lopes Iago José Vitral Rezende, et al, 2021, Influences of biosilica content from Amazonian freshwater sponge on calcium phosphates, Journal of the Australian Ceramic Society, 57(1), 55, 10.1007/s41779-020-00509-6
- 16.Jodati Hossein, Yılmaz Bengi, Evis Zafer, 2020, A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features, Ceramics International, 46(10), 15725, 10.1016/j.ceramint.2020.03.192
- 17.Zhang Yue, Zhang Leilei, Nie Hongwen, Wang Mengting, Dong Zhijie, 2022, In situ grown carbon nanotube-Fe3O4-dicalcium phosphate dehydrate composite coating on carbon fibres for bone tissue application, Materials Technology, 37(14), 3122, 10.1080/10667857.2022.2127641
- 18.Copete Hamilton, López Esperanza, Baudin Carmen, 2023, Synthesis and characterization of B-type carbonated hydroxyapatite materials: Effect of carbonate content on mechanical strength and in vitro degradation, Boletín de la Sociedad Española de Cerámica y Vidrio, (), 10.1016/j.bsecv.2023.12.001
- 19.Yazdanian Mohsen, Alam Mostafa, Abbasi Kamyar, Rahbar Mahdi, Farjood Amin, et al, 2022, Synthetic materials in craniofacial regenerative medicine: A comprehensive overview, Frontiers in Bioengineering and Biotechnology, 10(), 10.3389/fbioe.2022.987195
- 20.Erceg Ina, Selmani Atiđa, Gajović Andreja, Panžić Ivana, Iveković Damir, et al, 2020, Calcium phosphate formation on TiO2 nanomaterials of different dimensionality, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 593(), 124615, 10.1016/j.colsurfa.2020.124615
- 21.Agnes Celine J., Murshed Monzur, Takada Adrien, Willie Bettina M., Tabrizian Maryam, 2023, A 6-bromoindirubin-3′-oxime incorporated chitosan-based hydrogel scaffold for potential osteogenic differentiation: Investigation of material properties in vitro, International Journal of Biological Macromolecules, 227(), 71, 10.1016/j.ijbiomac.2022.12.130