Abstract
Silver sulfadiazine is a topical medicine that belongs to sulfa antibiotics class of drugs and used in treating wound infections in burn patients. The aim of this study was to determine the effect of Consciousness Energy Healing Treatment (the Trivedi Effect®) on the various properties of silver sulfadiazine with the help of modern analytical techniques. The sample was divided into two parts; the first part was not given any treatment and considered as a control sample, while the second part was provided the Consciousness Energy Healing Treatment by the Biofield Energy Healer, Gopal Nayak remotely, named as the treated sample. The powder XRD data showed significant alterations in the peak intensities of the treated sample ranging from-30.71% to 47.54% compared to the control sample. The crystallite size was altered ranging from -78.12% to 1.47%; and the average crystallite size was significantly reduced by 31.62% in the treated sample compared to the control sample. The particle sizes were decreased in the treated sample by 12.75%(d10), 4.98%(d50), 0.89%(d90), and 2.92%{D(4,3)}; thus, the specific surface area was significantly increased by 17.31% compared with the control sample. The latent heat of fusion and latent heat of decomposition were profoundly increased by 24.62% and 156.28%, respectively in the treated sample compared to the control sample. The total weight loss was increased by 3.08% and the residue amount was reduced by 4.44% in the treated sample compared to the control sample. Thus, the Trivedi Effect®-Consciousness Energy Healing Treated sample might form a new polymorph of silver sulfadiazine that possesses reduced particle size and improved thermal properties compared to the untreated sample. Therefore, the Biofield Energy treated sample can be used in nutraceutical/pharmaceutical formulation, which would show a better bioavailability and therapeutic response against various infections in comparison to the control sample.
Author Contributions
Academic Editor: Syed Najmul Hejaz Azmi, Department of Applied Sciences, Chemistry Section, Higher College of Technology, P. O. Box 74, Al-Khuwair-133, Muscat, Sultanate of Oman, Oman.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Gopal Nayak, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Silver sulfadiazine is a topical medicine that belongs to sulfa- antibiotics class of drugs. It is used as an adjuvant in treating and preventing wound infections in burn patients. Silver sulfadiazine works on the bacteria by stopping their growth on the open wound; thereby decrease the risk of their spreading to the surrounding skin or to the blood where they may cause sepsis 1. Silver ions are considered as a biocide that has the ability to binds to a broad range of targets. Such targets include the nucleophilic amino acids, amino, sulfhydryl, phosphate, imidazole, and carboxyl groups present in the proteins. Moreover, the binding to such sites of proteins causes enzyme inhibition by the process of protein denaturation 2. Also, silver binds to the proteins present on the surface membranes and thus causes proton leaks in the membrane, which ultimately leads to the death of the cell. Besides, sulfadiazine works as a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), which inhibited the reaction that is vital in the folic acid synthesis 3, 4. Although silver sulfadiazine is used topically; however, it is poorly soluble and has limited penetration through intact skin 5. In recent days, the studies have been done on the physicochemical properties of the drug that mainly impart their effect on the absorption and bioavailability parameters of the formulation 6. Thus, this study was also done with emphasizing the techniques that may improve these parameters ofsilver sulfadiazine.
The Biofield Energy Healing Treatment (the Trivedi Effect®) has been considered these days as a novel approach due to its considerable impact on the physicochemical and thermal behaviour of various compounds 7, 8, 9. The Trivedi Effect® is considered as a scientifically proven phenomenon which involves the principle that a person can harness the inherently intelligent energy from its surroundings and transmit it in an object(s) (living/non-living) through the possible mediation of neutrinos 10. This kind of energy is known as Biofield Energy that is possessed by every living organism surrounding the body, and it is infinite and para-dimensional electromagnetic field. The Biofield Energy (Putative Energy Fields) based Healing Therapies have been reported by various studies due to their significant outcomes against various medical conditions 11. Therefore, the National Institutes of Health (NIH) and the National Center for Complementary and Alternative Medicine (NCCAM) included such Energy therapies under the category of Complementary and Alternative Medicine (CAM) and is accepted by the most of the U.S. population 12, 13. The Trivedi Effect®-Consciousness Energy Healing Treatment has also been known for its ability to alter the characteristic properties of the organic compounds 14, 15, 16, metals and ceramic 17, 18, nutraceuticals/pharmaceuticals 19, 20, and in the field of microbiology 21, 22, 23, skin health 24, 25, biotechnology 26, 27, bone health 28, 29, 30, cancer science research 31, 32, and crops 33, 34. Hence, the current study was designed with the aim to analyze the effect of the Trivedi Effect®-Consciousness Energy Healing Treatment on the physicochemical and thermal properties of silver sulfadiazine using modern analytical techniques.
Materials and Methods
Chemicals and Reagents
Silver sulfadiazine was purchased from Tokyo Chemical Industry Co., Ltd., Japan. All other chemicals used during the experiments were of analytical grade available in India.
Consciousness Energy Healing Treatment Strategies
The study involved dividing the silver sulfadiazine test sample into two parts. The first part was considered as a control sample and no Biofield Energy Treatment was given to it. Besides, the second part of the sample was treated with the Trivedi Effect®-Energy of Consciousness Healing Treatment by the renowned Biofield Energy Healer, Gopal Nayak, India, remotely under standard laboratory conditions for 3 minutes and known as the Biofield Energy Treated sample. Later, the control sample was treated with a “sham” healer (did not have any knowledge about the Biofield Energy Treatment) for comparison purpose. Consequently, the control and Biofield Energy Treated samples were kept in sealed conditions and characterized using modern analytical techniques.
Characterization
The powder X-ray diffraction (PXRD), particle size analysis (PSA), thermogravimetric analysis (TGA)/differential thermogravimetric analysis (DTG), and differential scanning calorimetry (DSC) analysis of silver sulfadiazine were performed. The powder XRD analysis of silver sulfadiazine powder sample was performed with the help of Rigaku MiniFlex-II Desktop X-ray diffractometer (Japan) 35, 36. The average size of crystallites was calculated from powder XRD data using the Scherrer’s formula (1)
G = kλ/βcosθ(1)
Where G is the crystallite size in nm, k is the equipment constant (0.94), λ is the radiation wavelength (0.154056 nm for Kα1 emission), β is the full-width at half maximum, and θ is the Bragg angle 37.
The PSA was performed using Malvern Mastersizer 2000, from the UK with a detection range between 0.01 µm to 3000 µm using the wet method. Similarly, the DSC analysis of silver sulfadiazine was performed with the help of DSC Q200, TA Instruments. The TGA/DTG thermograms of silver sulfadiazine were obtained with the help of TGA Q50 TA instruments 38, 39.
The % change in particle size, specific surface area (SSA), peak intensity, crystallite size, melting point, latent heat, weight loss and the maximum thermal degradation temperature (Tmax) of the Biofield Energy Treated sample was calculated compared with the control sample using the following equation 2:
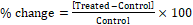 (2)
(2)
Results and Discussion
Powder X-ray Diffraction (PXRD) Analysis
The Biofield Energy Treated sample showed sharp and intense peaks in its diffractogram that are similar to the diffractogram of the control sample, representing the crystalline nature of both the samples. However, the Bragg’s angles (2q) of these peaks of the treated sample were observed to differ from the Bragg’s angles of the peaks of the control sample (Figure 1) in the PXRD diffractogram. The highest peak intensity in the diffractograms of the control and the treated sample was present at 2θ equal to 10.08° and 10.37º, respectively (Table 1, entry 2). Besides, the intensities of the peaks of the Biofield Energy Treated sample were found to be significantly altered ranging from -30.71% to 47.54% in comparison to the control sample. Also, the crystallite sizes of the treated silver sulfadiazine sample were observed to be altered significantly compared to the control sample. The significant decrease in crystallite size was observed for most of the peaks ranging from 2.34% to 78.12%; however the crystallite size at position 2θ equals to 20.69º was slightly increased by 1.47% (Table 1, entry 7), as compared to the control sample. The average crystallite size of the treated silver sulfadiazine sample (307.36 nm) was also decreased by 31.62% in comparison to the control sample (449.50 nm).
Figure 1.PXRD diffractograms of the control and Biofield Energy Treated silver sulfadiazine.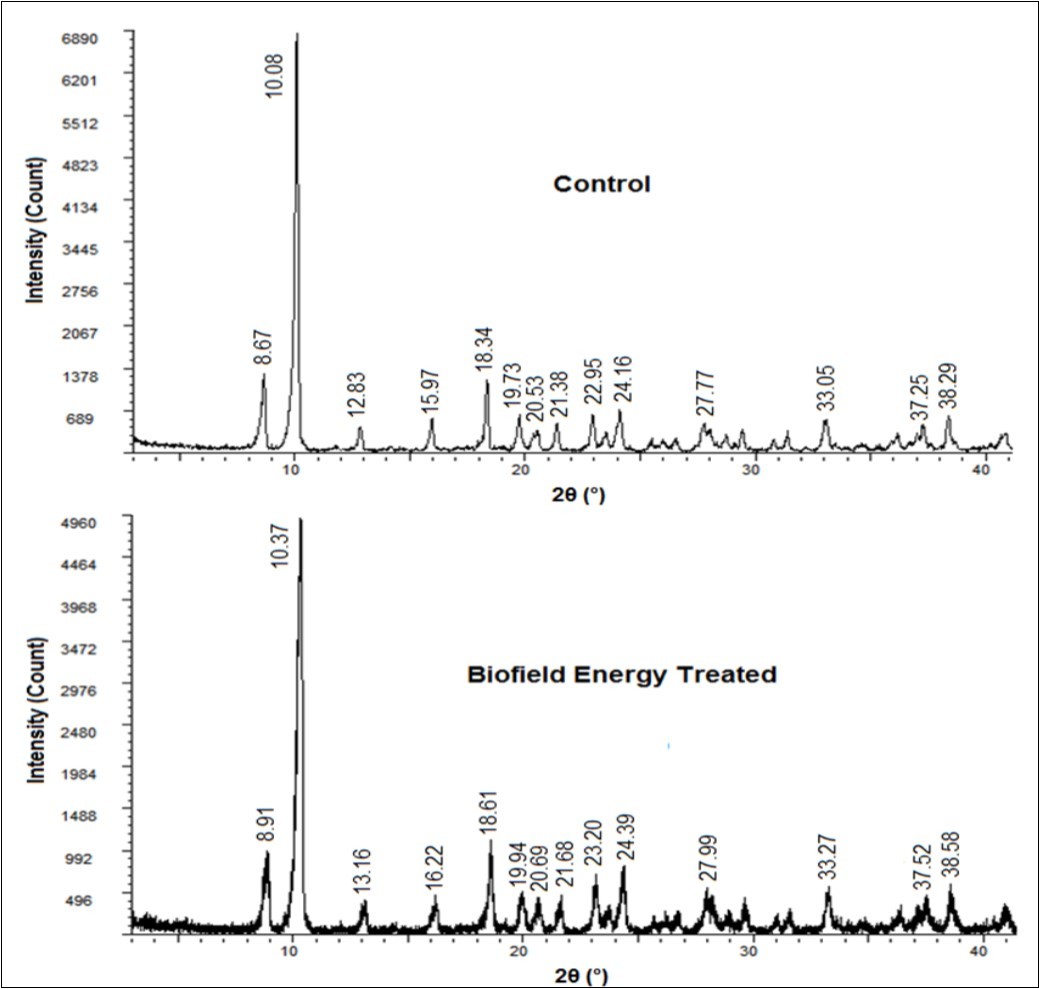
| Entry No. | Bragg angle (°2θ) | Peak Intensity (%) | Crystallite size (G, nm) | |||||
| Control | Treated | Control | Treated | % change a | Control | Treated | % change b | |
| 1 | 8.67 | 8.91 | 241 | 167 | -30.71 | 564 | 272 | -51.77 |
| 2 | 10.08 | 10.37 | 1059 | 968 | -8.59 | 536 | 304 | -43.28 |
| 3 | 12.83 | 13.16 | 57 | 51 | -10.53 | 522 | 350 | -32.95 |
| 4 | 15.97 | 16.23 | 63 | 61 | -3.17 | 474 | 345 | -27.22 |
| 5 | 18.34 | 18.61 | 150 | 171 | 14.00 | 621 | 399 | -35.75 |
| 6 | 19.73 | 19.94 | 76 | 75 | -1.32 | 348 | 310 | -10.92 |
| 7 | 20.53 | 20.69 | 64 | 65 | 1.56 | 272 | 276 | 1.47 |
| 8 | 21.38 | 21.68 | 55 | 49 | -10.91 | 478 | 353 | -26.15 |
| 9 | 22.95 | 23.20 | 83 | 89 | 7.23 | 463 | 362 | -21.81 |
| 10 | 24.16 | 24.39 | 113 | 118 | 4.42 | 368 | 327 | -11.14 |
| 11 | 27.77 | 27.99 | 138 | 132 | -4.35 | 175 | 167 | -4.57 |
| 12 | 33.05 | 33.27 | 97 | 87 | -10.31 | 385 | 376 | -2.34 |
| 13 | 37.25 | 37.52 | 61 | 90 | 47.54 | 681 | 149 | -78.12 |
| 14 | 38.29 | 38.58 | 86 | 75 | -12.79 | 406 | 313 | -22.91 |
It is studied in various researches that the peak intensity of each diffraction face varied based on the alteration in the crystal morphology of the crystalline compound 40. Such changes in the intensities of the peak and the corresponding alterations in the crystallite sizes of the treated sample indicate that the crystal morphology might be affected due to the Biofield Energy Treatment, compared to the untreated sample. Also, the changes in the PXRD pattern denote polymorphic transitions taken place in the compound 41, 42. Various scientific studies confirm the impact of different polymorphic forms of any drug on its therapeutic efficacy, bioavailability, and toxicity 43, 44. Thus, the Trivedi Effect® Treated sample might be considered to form a new polymorphic form of silver sulfadiazine that may offer better performance when used in the pharmaceutical formulations in comparison to the untreated sample.
Particle Size Analysis (PSA)
The control and Biofield Energy Treated sample were analysed for their particle size distribution and the results were presented in Table 2. The control silver sulfadiazine showed particle size distribution at d10, d50, d90, and D(4,3) as 7.92 µm, 30.09 µm, 93.61 µm, and 43.51 µm, respectively. On the other hand, the particle size values of the treated sample were observed as 6.91 µm, 30.49 µm, 92.78 µm, and 42.24 µm at d10, d50, d90, and D(4,3), respectively. Hence, the Biofield Energy Treated sample showed a decrease in particle size values by 12.75%, 4.98%, 0.89%, and 2.92% at d10, d50, d90, and D(4,3), respectively compared to the control silver sulfadiazine sample. The reduction in particle sizes resulted in a significant increase in the specific surface area of the treated sample (0.61m2/g) by 17.31% as compared with the control sample (0.52m2/g). Thus, it could be presumed that the Trivedi Effect® -Consciousness Energy Healing Treatment might help in reducing the particle size of the treated sample by acting like an external force 45. Besides, it is established in various studies that the reduced particle size ensures increased surface area that ultimately helps to improve the bioavailability of the drug 46, 47, 48. Hence, the Biofield Energy Treated silver sulfadiazine might offer improved absorption and in comparison to the untreated sample.
Table 2. Particle size distribution of the control and Biofield Energy Treated silver sulfadiazine.| Parameter | d10 (µm) | d50 (µm) | d90 (µm) | D(4,3)(µm) | SSA(m2/g) |
| Control | 7.92 | 32.09 | 93.61 | 43.51 | 0.52 |
| Biofield Treated | 6.91 | 30.49 | 92.78 | 42.24 | 0.61 |
| Percent change* (%) | -12.75 | -4.98 | -0.89 | -2.92 | 17.31 |
Differential Scanning Calorimetry (DSC) Analysis
The DSC analysis of the control and Biofield Energy Treated sample has been done (Table 3 and Figure 2) for characterizing the thermal behavior of both the samples. The DSC thermograms of both the samples showed two peaks among which, one was endothermic while the other was exothermic in nature. The thermograms of the control and treated sample showed the sharp endothermic peak at 262.90°C and 262.55°C, respectively that is considered as their melting point (Figure 2). The further analysis revealed a slight decrease in the melting point of the treated sample by 0.13% in comparison to the control sample (Table 3).
Figure 2.DSC thermograms of the control and Biofield Energy Treated silver sulfadiazine.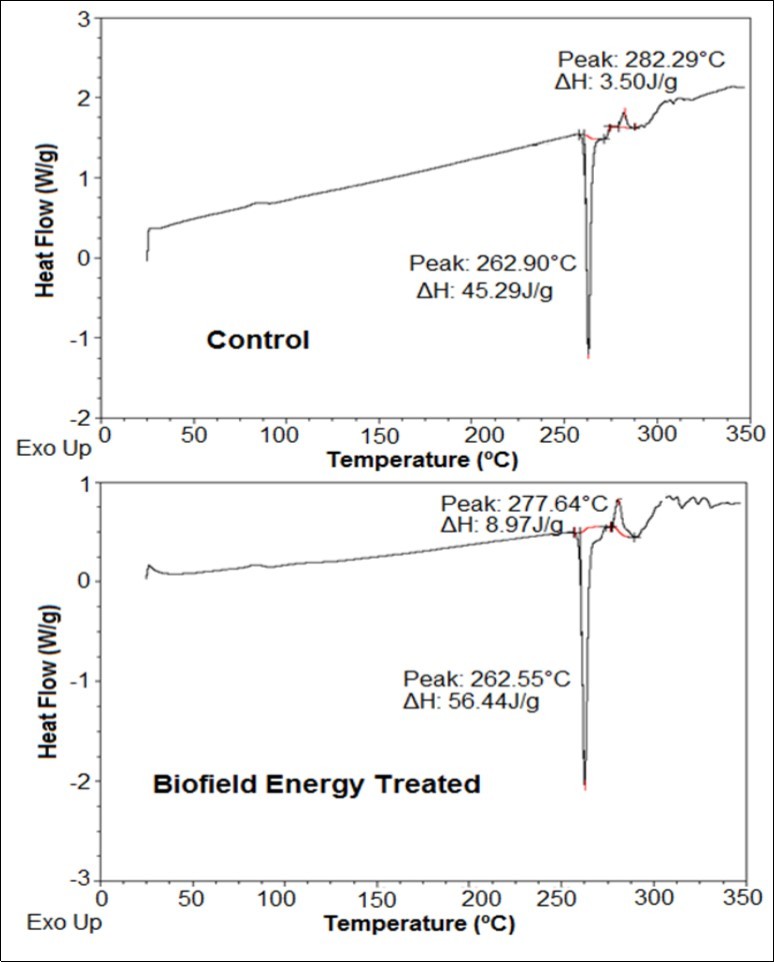
| Sample | Melting point (°C) | ∆H(J/g) | Decomposition temperature | ∆H(J/g) |
| Control Sample | 262.90 | 45.29 | 282.29 | 3.50 |
| Biofield Energy Treated | 262.55 | 56.44 | 277.64 | 8.97 |
| % Change* | -0.13 | 24.62 | -1.65 | 156.28 |
However, the latent heat of fusion (∆Hfusion) of the treated silver sulfadiazine was found to be significantly increased by 24.62% compared with the control sample (Table 3). Besides, the exothermic peak observed in the thermograms of both the samples represents the decomposition temperature of silver sulfadiazine. Although, the decomposition temperature of the Biofield Energy Treated sample was slightly decreased by 1.65%, however, the corresponding ∆H was significantly increased by 156.28% that could be due to the disruption in the molecular chains present in the crystal structure 45. Hence, it is presumed that the Biofield Energy Treatment might disrupt the crystal structure of silver sulfadiazine that ultimately resulted in the decreased melting point of the Biofield Energy Treated sample in comparison to the control sample.
Thermal Gravimetric Analysis (TGA)/ Differential Thermogravimetric Analysis (DTG)
The TGA thermograms of the control and Biofield Energy Treated samples are shown in Figure 3 and the data were presented in Table 4. The data reported the increase in the total weight loss of the treated sample by 3.08% and the residue amount was decreased by 4.44% compared with the control sample (Table 4).
Table 4. TGA/DTG data of the control and Biofield Energy Treated samples of silver sulfadiazine.| Sample | TGA | DTG | |
| Total weight loss (%) | Residue % | Tmax (°C) | |
| Control | 58.99 | 41.01 | 281.80 |
| Biofield Energy Treated | 60.81 | 39.19 | 277.10 |
| % Change* | 3.08 | -4.44 | -1.67 |
Figure 3.TGA thermograms of the control and Biofield Energy Treated silver sulfadiazine.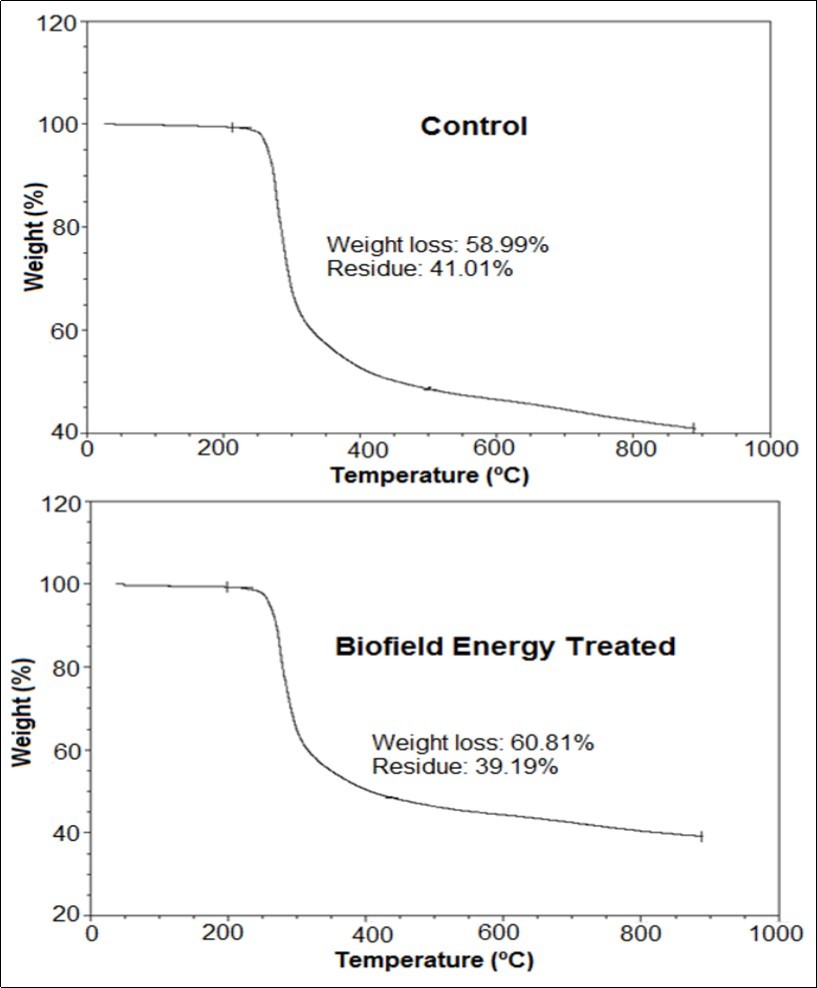
The DTG thermograms of both, the control and the treated silver sulfadiazine sample showed only one peak (Figure 4). The Tmax of the control sample was observed at 281.80°C, while the treated sample showed it at 277.10°C. Thus, the Biofield Energy Treated sample showed a slight reduction in the Tmax by 1.67% as compared with the control sample (Table 4). Overall, TGA/DTG revealed that the thermal stability of the Biofield Energy Treated silver sulfadiazine was altered compared with the control sample that might occur due to the reduction in the particle size of the sample 49.
Figure 4.DTG thermograms of the control and Biofield Energy Treated silver sulfadiazine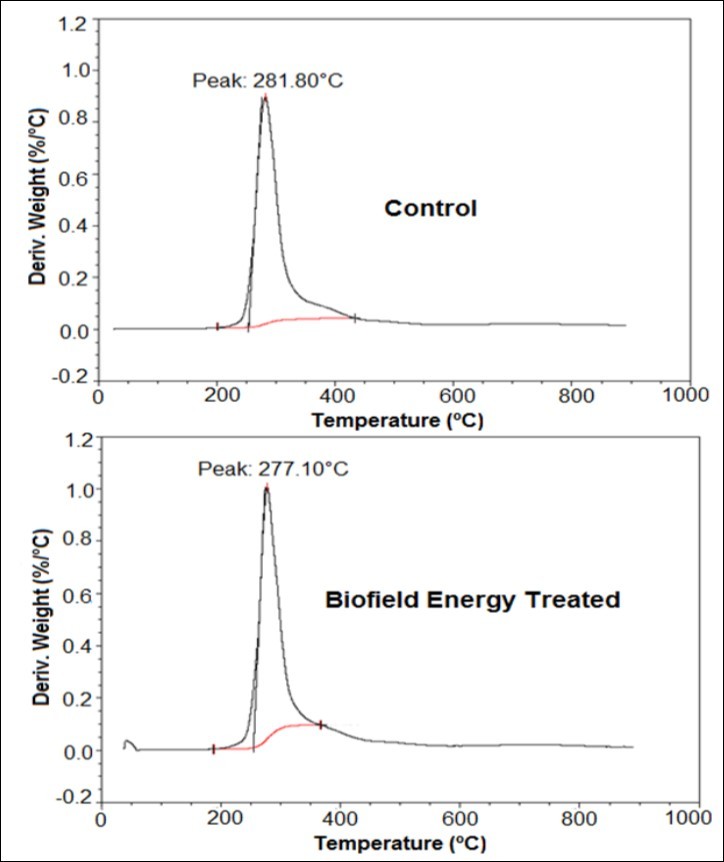
Conclusions
The Trivedi Effect®-Consciousness Energy Healing Treatment has a profound impact on various physicochemical and thermal properties of silver sulfadiazine. The powder XRD data showed significant alterations in the peak intensities of the Biofield Energy Treated sample ranging from -30.71% to 47.54% compared to the control sample. The crystallite size was altered ranging from -78.12% to 1.47%; and the average crystallite size was significantly reduced by 31.62% in the Biofield Energy Treated sample compared to the control sample. The particle sizes were decreased in the Biofield Energy Treated sample by 12.75%(d10), 4.98%(d50), 0.89%(d90), and 2.92%{D(4,3)}; thus, the specific surface area was significantly increased by 17.31% compared with the control sample. The ∆Hfusion and ∆Hdecomposition were profoundly increased by 24.62% and 156.28%, respectively in the Biofield Energy Treated sample compared to the control sample. The total weight loss was increased by 3.08% and the residue amount was reduced by 4.44% in the Biofield Energy Treated sample compared to the control sample. Overall, it is concluded that the Trivedi Effect®-Consciousness Energy Healing Treatment might have generated a novel polymorph of silver sulfadiazine that may show better bioavailability in comparison to the control sample. Hence, the Consciousness Energy Healing Treated silver sulfadiazine would be more useful in designing the formulations with improved efficacy against burning infections.
Acknowledgements
The authors are grateful to Central Leather Research Institute, SIPRA Lab. Ltd., Trivedi Science, Trivedi Global, Inc., Trivedi Testimonials, and Trivedi Master Wellness for their assistance and support during this work.
References
- 1.Marx J, Walls R, Hockberger R. (2013) Rosen's Emergency Medicine-Concepts and Clinical Practice, Volume 2, 8th edn, Elsevier Health Sciences. , US
- 2.Fox CL Jr. (1977) Pharmacology and clinical use of silver sulfadiazine and related topical antimicrobial agents. Pahlavi Med. , J 8, 45-64.
- 3.Harrison H N. (1979) Pharmacology of Sulfadiazine Silver Its Attachment to Burned Human and Rat Skin and Studies of Gastrointestinal Absorption and Extension. Arch Surg. 114, 281-285.
- 4.Wysor M S, Zollinhofer R E. (1972) On the Mode of Action of Silver Sulfadiazine. , Pathobiology 38, 296-308.
- 6.Charles H N, James E A, Milo G. (1971) Physiologic surface active agents and drug absorption VIII: Effect of bile flow on sulfadiazine absorption in the rat. , J Pharm Sci 60, 145-147.
- 7.Trivedi M K, Branton A, Trivedi D, Nayak G, Lee A C. (2016) Impact of biofield energy treated herbomineral formulation (The Trivedi Effect®) on mouse dendritic and splenocyte cells for modulation of pro-inflammatory cytokines. , International Journal of Immunology 4, 35-45.
- 8.Trivedi M K, Branton A, Trivedi D, Nayak G, Wellborn B D. (2017) Effect of the energy of consciousness (The Trivedi Effect®) on the structural properties and isotopic abundance ratio of magnesium gluconate using LC-MS and NMR spectroscopy. Advances in Biochemistry 5, 7-15.
- 9.Trivedi M K, Branton A, Trivedi D, Nayak G, Afaganis A E. (2017) An Impact of energy of consciousness (The Trivedi Effect®) on the physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. , Biomedical Sciences 3, 42-54.
- 10.Trivedi M K, TRR Mohan. (2016) Biofield energy signals, energy transmission and neutrinos. , American Journal of Modern Physics 5, 172-176.
- 11.Rubik B, Muehsam D, Hammerschlag R, Jain S. (2015) Biofield science and healing: history, terminology, and concepts. , Glob Adv Health Med 4, 8-14.
- 12.Barnes P M, Bloom B, Nahin R L. (2008) Complementary and alternative medicine use among adults and children: United States,2007. Natl Health Stat Report. 12, 1-23.
- 14.Trivedi M K, Branton A, Trivedi D, Nayak G, Sethi K K. (2016) Gas chromatography-mass spectrometry based isotopic abundance ratio analysis of biofield energy treated methyl-2-napthylether (Nerolin). , American Journal of Physical Chemistry 5, 80-86.
- 15.Trivedi M K, Branton A, Trivedi D, Nayak G, Bairwa K. (2015) Spectroscopic characterization of disodium hydrogen orthophosphate and sodium nitrate after biofield treatment. , J Chromatogr Sep Tech 6, 282.
- 16.Trivedi M K, Branton A, Trivedi D, Nayak G, Panda P. (2016) Evaluation of the isotopic abundance ratio in biofield energy treated resorcinol using gas chromatography-mass spectrometry technique. , Pharm Anal Acta 7, 481.
- 17.Trivedi M K, Tallapragada R M, Branton A, Trivedi D, Nayak G. (2015) Characterization of physical and structural properties of aluminum carbide powder: Impact of biofield treatment. , J Aeronaut Aerospace Eng 4, 142.
- 18.Trivedi M K, Patil S, Tallapragada R M. (2013) Effect of biofield treatment on the physical and thermal characteristics of vanadium pentoxide powders. , J Material Sci Eng. S 11, 001.
- 19.Trivedi M K, Tallapragada R M, Branton A, Trivedi D, Nayak G. (2015) Potential impact of biofield treatment on atomic and physical characteristics of magnesium. , Vitam Miner 3, 129.
- 20.Trivedi M K, Patil S, Shettigar H, Bairwa K, Jana S. (2015) Effect of biofield treatment on spectral properties of paracetamol and piroxicam. Chem Sci. , J 6, 98.
- 21.Trivedi M K, Branton A, Trivedi D, Nayak G, Charan S. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. , J Clin Med Genom 3, 129.
- 22.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. , J Antivir Antiretrovir 7, 083-088.
- 23.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis. 5, 189.
- 24.Kinney J P, Trivedi M K, Branton A, Trivedi D, Nayak G. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. , American Journal of Life Sciences 5, 65-74.
- 25.Singh J, Trivedi M K, Branton A, Trivedi D, Nayak G. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. , American Journal of Pharmacology and Phytotherapy 2, 1-10.
- 26.Trivedi M K, Patil S, Shettigar H, Bairwa K, Jana S. (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. , J Microb Biochem Technol 7, 203-206.
- 27.Nayak G, Altekar N. (2015) Effect of biofield treatment on plant growth and adaptation. , J Environ Health Sci 1, 1-9.
- 28.Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. , International Journal of Biomedical Engineering and Clinical Science 4, 6-14.
- 29.Lee A C, Trivedi K, Branton A, Trivedi D, Nayak G. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). , International Journal of Nutrition and Food Sciences 7, 30-38.
- 30.Stutheit M E, Trivedi K, Branton A, Trivedi D, Nayak G. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. , American Journal of Life Sciences 6, 13-21.
- 31.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. , J Integr Oncol 4, 141.
- 32.Trivedi M K, Patil S, Shettigar H, Gangwar M, Jana S. (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. , J Cancer Sci Ther 7, 253-257.
- 33.Trivedi M K, Branton A, Trivedi D, Nayak G, Gangwar M. (2015) Agronomic characteristics, growth analysis, and yield response of biofield treated mustard, cowpea, horse gram, and groundnuts. , International Journal of Genetics and Genomics 3, 74-80.
- 34.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C. (2015) Evaluation of plant growth, yield and yield attributes of biofield energy treated mustard (Brassica juncea) and chick pea (Cicer arietinum) seeds. Agriculture, Forestry and Fisheries. 4, 291-295.
- 36.Zhang T, Paluch K, Scalabrino G, Frankish N, Healy A M. (2015) Molecular structure studies of (1S,2S)-2-benzyl-2,3-dihydro-2-(1Hinden-2-yl)- 1H-. , inden-1-ol. J Mol Struct 1083, 286-299.
- 37.Langford J I, AJC Wilson. (1978) Scherrer after sixty years: A survey and some new results in the determination of crystallite size. , J Appl Cryst 11, 102-113.
- 38.Trivedi M K, Sethi K K, Panda P, Jana S. (2017) A comprehensive physicochemical, thermal, and spectroscopic characterization of zinc (II) chloride using Xray diffraction, particle size distribution, differential scanning calorimetry, thermogravimetric analysis/differential thermogravimetric analysis, ultravioletvisible, and Fourier transforminfrared spectroscopy. , International Journal of Pharmaceutical Investigation 7, 33-40.
- 39.Trivedi M K, Sethi K K, Panda P, Jana S. (2017) Physicochemical, thermal and spectroscopic characterization of sodium selenate using XRD, PSD, DSC, TGA/DTG, UV-vis, and FT-IR. , Marmara Pharmaceutical Journal 21(2), 311-318.
- 40.Inoue M, Hirasawa I. (2013) The relationship between crystal morphology and XRD peak intensity on CaSO4.2H2O. , J Crystal Growth 380, 169-175.
- 41.Raza K, Kumar P, Ratan S, Malik R, Arora S. (2014) Polymorphism: The phenomenon affecting the performance of drugs. , SOJ Pharm Pharm Sci 1, 10.
- 42.Brittain H G. (2009) Polymorphism in pharmaceutical solids in Drugs and Pharmaceutical Sciences, volume 192, 2nd Edn, Informa HealthcareUSA,Inc.,NewYork.
- 43.Censi R, Martino P D. (2015) Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. , Molecules 20, 18759-18776.
- 44.Blagden N, deMatas M, Gavan P T, York P. (2007) Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev. 59, 617-630.
- 45.Zhao Z, Xie M, Li Y, Chen A, Li G. (2015) Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. , Int J Nanomedicine 10, 3171-3181.
- 46.Loh Z H, Samanta A K, PWS Heng. (2015) Overview of milling techniques for improving the solubility of poorly water-soluble drugs. , Asian J Pharm 10, 255-274.
- 47.Khadkaa P, Roa J, Kim H, Kim I, Kim J T. (2014) Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. , Asian J Pharm 9, 304-316.
