Abstract
The aim of the study was to synthesize sub-100nm poly-ε-caprolactone nanoparticles (PCL NP), load them with the mycobacterial protein, ESAT 6 and study the resulting immune responses in CD4+ and CD8+ T cells when incubated with human peripheral blood monocyte derived macrophages that had internalized the PCL NP. The synthesized PCL NP were characterized for size, shape and charge. They were found to be about 60nm in size with spherical shape. MTT assay revealed that the particles were perfectly biocompatible when tested in vitro on THP1 human monocytic cell line. The particles had a slow protein release kinetics and did not degrade appreciably even after 30 days in buffer solution. ELISA was used to quantify the cytokine response of CD4+ and CD8+ T cells when incubated with the monocyte derived macrophages as antigen presenting cells. The result of antigen presentation assay revealed that the antigen loaded PCL NP enhanced Th1 and CD8+ T cell responses significantly compared to the pure antigen. Thus we conclude that PCL NP of 60nm size can be effectively tested as a vaccine adjuvant with resulting activation of Th1/Th2 immunity as well as cytotoxic T cell response.
Author Contributions
Academic Editor: Zhe-Sheng Chenz, Assistant Professor, Department of Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John’s University.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2014 Chandravilas Keshvan Prashant, et al.
 This is an open-access article distributed under the terms of theCreative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of theCreative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Tuberculosis, caused by the causative agent M.tuberculosis, has become a global scourge claiming almost 2 million lives per year and infecting almost 9 million people annually. The widely used TB vaccine, M.bovisBacille Calmette-Geurin (BCG) used to immunize infants and young children to prevent disseminated TB fails to prevent pulmonary (reactivation) TB in adolescents and adults. 1 Protective immunity against M.tuberculosis depends on the generation of a strong Th1 and CD8+ T cell response. 2 At least 12 TB vaccine candidates are in clinical trials which include recombinant live vaccines and viral vectored vaccines but majority are recombinant protein subunit vaccines. Early secreted antigenic target, ESAT-6 has been identified as a highly immunogenic mycobacterial antigen that is conserved in a large number of mycobacterial strains. It is encoded by genes located in the RD1 region in the mycobacterial genome. 3 This region is known to be deleted in all strains of BCG. Hence this secreted mycobacterial protein is an ideal antigen for subunit vaccine development against TB with suitable adjuvants. Studies have indicated that this secreted protein gains entry into the cytosol from the bacterial phagosomes and gets presented on both MHC class-I and MHC class II molecules.
Enhancing the cross-presentation of ESAT 6 antigen may help to develop a strong CD8+ T cell response against M.tuberculosis. Many studies have indicated that particulate antigens are cross-presented more efficiently than the antigen alone. 4,5,6,7,8 With this aim, we entrapped ESAT 6 antigen in poly-ε-caprolactone (PCL) nanoparticles of about 60nm and studied the enhancement of cross-presentation of this antigen due to the nanoparticles.
Poly-ε-caprolactone has been reported as an antigen delivery system by some investigators. Gamazo et al encapsulated a hot saline antigenic extract (HS) from Brucella ovis in poly-epsilon-caprolactone microparticles, and tested them as a vaccine against B.ovis and B.abortus infections in mice. 9 The vaccine administered either subcutaneously or orally protected mice against B.ovis infection. Subcutaneous but not oral administration in BALB/c mice of the HS-PCL induced high amounts of IFN-gamma and IL-2 but low quantities of IL-4 suggesting a combined Th1/Th2 cellular immune response. Alpar et al tested diphtheria toxoid (DT)-loaded polycaprolactone nanoparticles as mucosal vaccine delivery systems (38).10 Poly-(epsilon-caprolactone) (PCL), a poly (lactide-co-glycolide) (PLGA)-PCL blend and co-polymer nanoparticles encapsulating diphtheria toxoid (DT) were investigated for their potential as a mucosal vaccine delivery system. PCL nanoparticles induced DT serum specific IgG antibody responses significantly higher than PLGA and it was different according to the route of administration, indicated by the differential levels of IL-6 and IFN-gamma. Almeida et al studied the humoral, cellular and mucosal immune responses to S.equi antigens encapsulated or adsorbed onto poly-epsilon-caprolactone nanospheres in mice. 11 Strangles is a bacterial infection of the Equidae family that affects the nasopharynx and draining lymph nodes, caused by Streptococcus equi subspecies equi. Particles were produced by a double (w/o/w) emulsion solvent evaporation technique and contained mucoadhesive polymers (alginate or chitosan) and absorption enhancers (spermine, oleic acid). Their intranasal administration increased the immunogenicity and mucosal immune responses to the antigen, particularly those constituted by the mucoadhesive polymers. They concluded that PCL nanospheres are potential carriers for the delivery of S.equi antigens to protect animals against strangles.
Dixit et al synthesized PCL microspheres for delivery of recombinant hepatitis B surface antigen (rHBsAg). 12 Immunization with HBsAg PCL microspheres caused significant secretion of IFN-γ and IL-2 as well as IgG response in BALB/c mice compared to the conventional alum adjuvant following a single intramuscular immunization.
Materials and Methods:
Preparation of Poly-Ε-Caprolactone Nanoparticles:
Poly-ε-caprolactone (MW 14 KD), pluronic® F127 and acetonitrile were procured from SIGMA-ALDRICH (St. Louis, MO). PCL Nanoparticles were synthesized by double emulsion system with Pluronic® 127 as surfactant. Briefly, 100mg of surfactant was added in 20 ml of water (solution A) and stirred for 1 hour. In another solution (solution B), 100mg of PCL was dissolved in 5ml solvent (Acetonitrile) and stirred for 1 hour. 60µl of ESAT 6 (2 mg/ml stock) was added to solution B under constant stirring. Immediately, polymer loaded with protein (solution B) was added to solution A at a slow rate. The system was left under constant stirring over night at 37°C until the solvent evaporated.
Characterization of PCL NP
The size and morphology of the nanoparticles were determined by transmission electron microscopy (TEM). One drop of the aqueous solution of PCL NP followed by one drop of 1% phosphotungstic acid was put on a formvar-coated copper grid and then air-dried. The dried grid was examined under a Philips Morgagni 268 electron microscope (Electron Microscopy Facility, All India Institute of Medical Sciences, New Delhi, India).
The Malvern Zetasizer 3000HS was used to determine the size distribution and zeta potential of PCL NP at 25°C. Size distribution was also determined using Nanosight™ LM 20.
Cell Culture
Human monocytic cell line THP1 was obtained from NCCS, Pune, India and used for PCL NP uptake and viability assay.
Confluent cells were sub-cultured and maintained at 37°C in Rosewell Park Memorial Institute (RPMI) medium SIGMA-ALDRICH (St. Louis, MO) under standard conditions. Media was supplemented with 10% fetal calf serum (Hyclone, Logan, UT), and antibiotic SIGMA-ALDRICH (St. Louis, MO) containing 50 U/mL of penicillin and 50 mg/mL of streptomycin and actinomycin.
MTT Assay for Cell Viability
MTT assay was done as previously described.13 THP1 cells were seeded at 1 x 103 cells per well and grown in 96-well plates until sub-confluent. Sterile aqueous solution of PCL NP dissolved in PBS (Ph 7.2) were then added to the cells at a concentration of 500µg/mL and incubated for 3h, 6h, 9h, 12h, 24h and 48h. Percentage viability of the cells was calculated as the ratio of mean absorbance of triplicate readings with respect to mean absorbance of control wells:
Cell viability = (I sample /I control ) × 100.
Protein Release Kinetics:
10mg of ESAT 6 loaded PCL NP (60nm) was dissolved in 1ml of either PBS (pH7.2), PBS (pH 4.0) or double distilled water (Ph 6.0) and kept at 37°C for 7, 14, 21, 28 and 30 days. After the indicated time points, the microfuge tubes were centrifuged at 15,000rpm and the supernatants were assayed for protein content using Bradford assay (Amresco, OH, USA). Values were obtained from standard curve obtained with bovine serum albumin (BSA) as reference protein. Void PCL NP were used as negative controls.
Loading Efficiency:
10mg of lyophilized PCL nanoparticles was dissolved in 1:1 ratio of Dichloromethane: Water. The sample was vortexed for 2 minutes and centrifuged at 8,000rpm for 10 minutes. The supernatant was collected and 10µl of it was added to 90µl of NaCl (0.15N) solution. The solution was made to 1ml by adding 900µl of Bradford Solution. OD was read at 595nm using a spectrophotometer (Shimadzu, MD, USA). Values were obtained from standard curves obtained with BSA as reference protein. Loading efficiency was calculated as:
[ESAT 6 TOTAL – ESAT 6 SUPERNATANT /ESAT 6 TOTAL ] x 100
Where ESAT 6TOTAL is the protein added during synthesis of the PCL NP and ESAT 6SUPERNATANT is the protein content in the supernatant.
Antigen Presentation Assay
10ml blood was drawn from healthy human volunteers and mononuclear cells were separated over ficoll hypaque gradient SIGMA-ALDRICH (St. Louis, MO)). Monocyte-derived macrophages were obtained and used in antigen presentation assay as previously described.14 For isolation of autologous T cells, CD4+ and CD8+ T cells were isolated by negative sorting using magnetic sorting as per company’s instructions (Invitrogen, Carlsbad, CA).
ESAT 6 loaded PCL NP was used at a concentration of 100µg/ml while the same concentration of pure ESAT 6 served as positive control. Void PCL NP and PBS served as negative control. Culture supernatants were then collected for ELISA of IL-4, IL-10, IFN gamma and IL-12 for CD4+ T cell response while IL-2 was assayed for CD8+T cell response.
Enzyme Linked Immunosorbent Assay (ELISA):
ELISA was done as per manufacturer’s instructions (PEPROTECH Inc NJ 08553 USA). Experiments were done in triplicates and average of six different experiments was taken for analysis.
Results
PCL NP were successfully synthesized in the size range of 61.2 ±28.6nm (mean±SD) and the sizes were determined using transmission electron microscopy (TEM) (Figure 1), dynamic light scattering (DLS) (Figure 1) and through Nanosight™ LM 20 (Figure 1) which uses a diffusion based method for size measurements. Zeta potential of 60nm PCL NP particles was-3±3.2mV. They were entrapped with antigen of interest viz ESAT 6.
Figure 1.A) TEM image of 60nm PCL NP revealing the spherical shape of the particles. The particles do not show aggregation and are well dispersed. B) DLS data of size distribution of PCL NP in solution. C) Size measurement of PCL NP by Nanosight. Mean particle size is 66nm.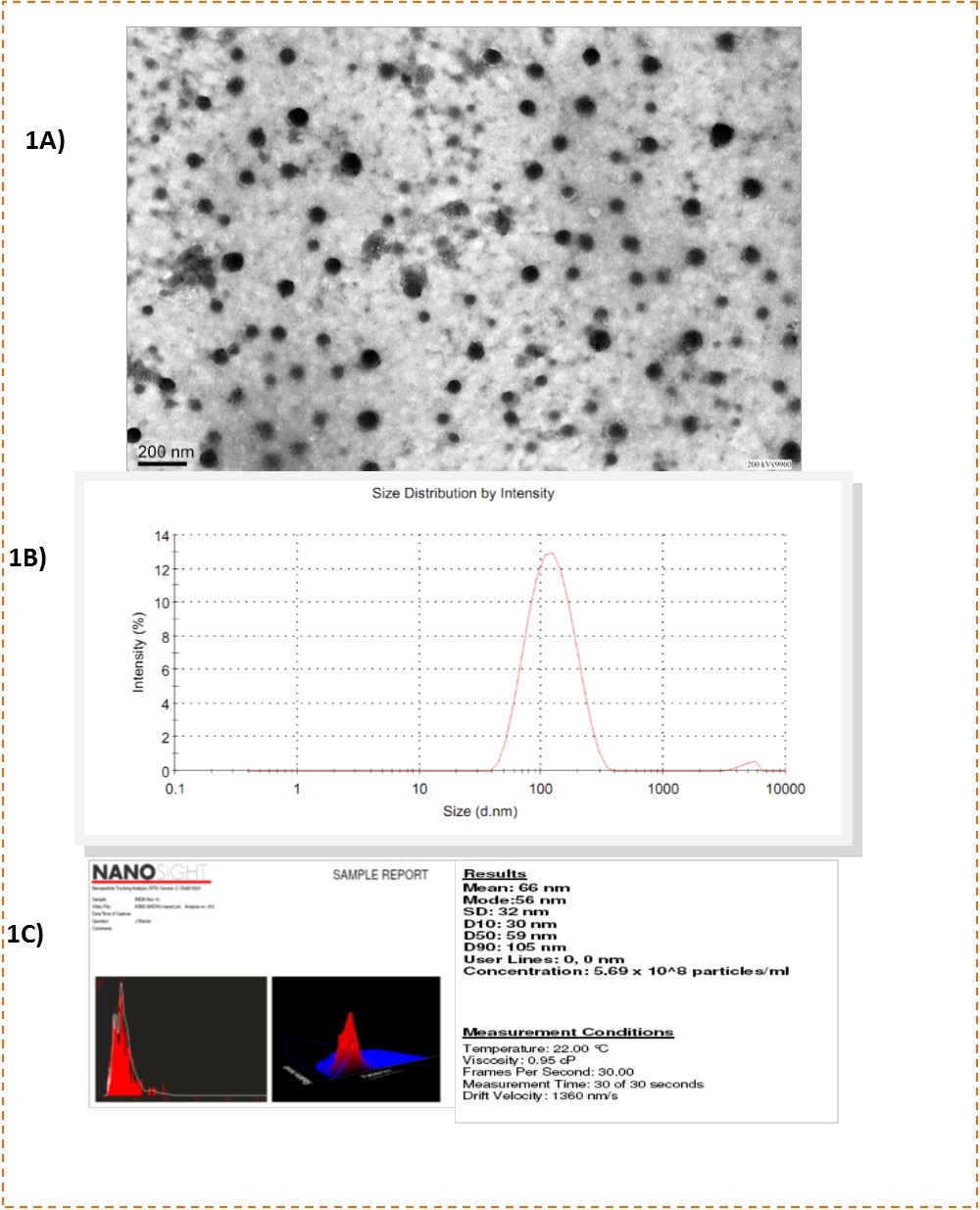
60nm PCL NP had an antigen loading efficiency of 61.66±16.96%. Degradation kinetics of PCL NP was studied with scanning electron microscopy (SEM) and is indicated in Figure 2.. The SEM images reveal that 60nm PCL NP do not degrade appreciably and have a spherical morphology even at 30 days. Protein release from the particles is shown in Figure 2.
Figure 2.A) SEM image of 60nm PCL NP after 30 days in PBS buffer (Ph 7.2). The particles retain their spherical morphology with some amount of swelling showing that they are stable in solution. B) Protein release kinetics of PCL NP in PBS buffer at different pH. The graph shows sustained and slow release pattern of ESAT 6 from the degrading PCL NP.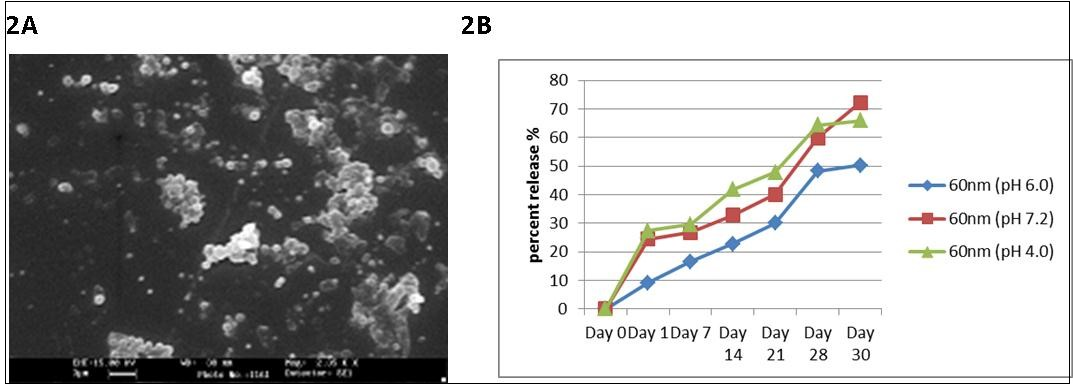
Cell viability assay done on THP1 cell line with MTT assay showed that the synthesized PCL NP were perfectly biocompatible (Figure 3) with viability of 99.34±0.66% for 60nm PCL NP. Figure 4 shows THP1 cells with internalized rhodamine labelled 60nm PCL NP at 6h incubation.
Figure 3.MTT Assay indicating percent viability of THP1 cells incubated for the defined time points with void PCL NP. Viability of the cells is nearly 99% which shows that the particles are non-toxic to the cells.
Figure 4.Rhodamine B labelled PCL NP internalized by THP1 cells after 6hrs incubation.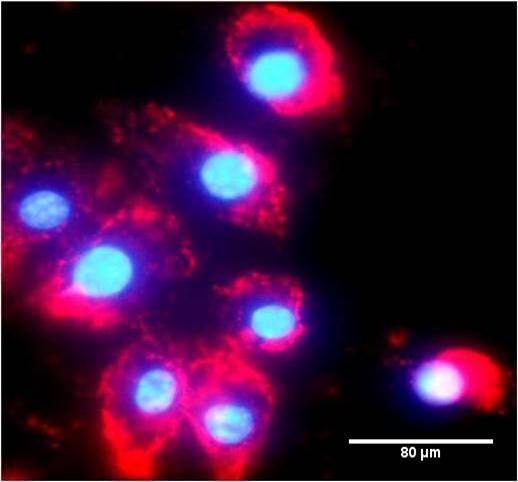
When ESAT 6 entrapped 60nm PCL NP were used in the antigen presentation assays, CD4+ T cells produce IFN gamma (p=0.0009) and IL-4 (p=0.002) in significant amounts (Figure 5) compared to the negative control and pure ESAT 6 antigen as positive control. CD8+ T cell response is also efficiently elicited by 60nm PCL NP as indicated by IL-2 secretion (p=0.0041) (Figure 6).
Figure 5.Cytokines secreted by CD4+ T cells in response to antigen presentation by monocyte derived macrophages. Data reveals the enhanced cytokine secretion by antigen loaded 60nm PCL NP compared to pure ESAT 6 antigen. A) IFN gamma secretion B) IL-4 secretion.
Figure 6.IL-2 secretion by CD8+ T cells incubated with monocyte derived macrophages after they have engulfed ESAT 6 loaded 60nm PCL NP.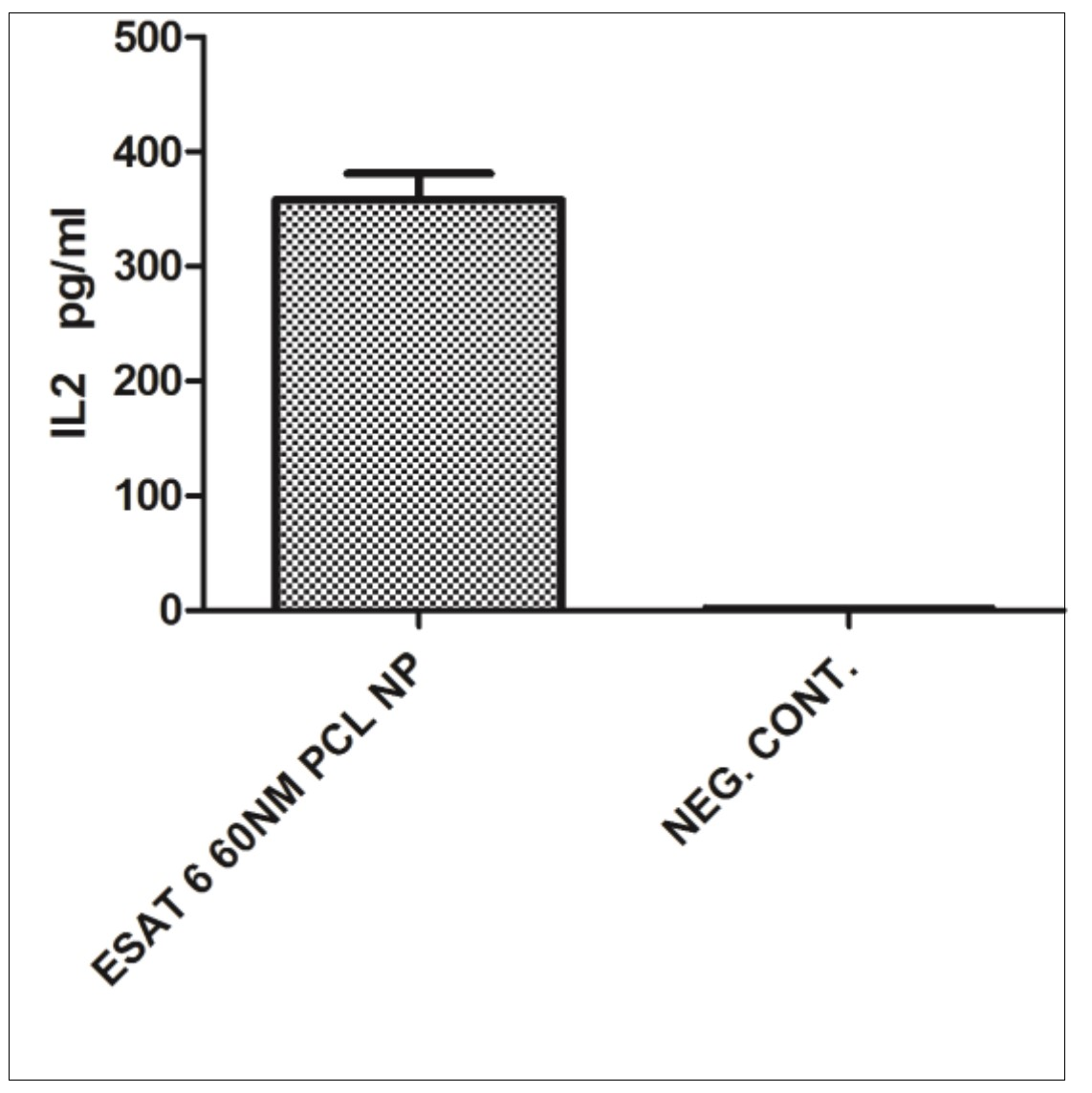
Discussion
The present in vitro study was carried out to investigate the adjuvant properties of PCL NP and see the effect of PCL NP on helper T cell polarization and cross-presentation of ESAT 6, loaded in the NPs and incubated with human peripheral blood monocyte derived macrophages as the APCs. PCL was chosen for the study as it is an FDA approved polymer and degrades at much slower rates than other popular polymers like PLA/PLGA making it ideal for slow and prolonged release of antigens.
The synthesized PCL NP had an average size of 61.2±28.6nm and were perfectly biocompatible. Protein release kinetics from the PCL NP show a slow and sustained release pattern. SEM images reveal that the particles have stable morphology and do not degrade appreciably even at 30 days of incubation in buffer.
The results of antigen presentation assay reveals that PCL NP enhance antigen cross presentation. The pure antigen elicits both IFN gamma and IL-4 responses in CD4+ T cells. But entrapping ESAT 6 in PCL NP and delivering them to macrophages resulted in enhanced production of both IFN gamma and IL-4 by CD4+ T cells. Importantly, antigen cross presentation is also enhanced by the PCL NP as revealed by increased amounts of IL-2 production by CD8+ T cells.
Thus an enhanced Th1/Th2 response is generated by ESAT 6 entrapped PCL NP compared to the pure antigen alone. The general trend for vaccine development has been to identify novel antigens that could provide long-lasting immunity but what has been neglected for long is the method for inducing strong immune responses against such antigens. Successful vaccination depends on the use of adjuvants.15
Usually, most of the antigens are weak immunogens and in the event of their being strong immunogens, the use of a carrier vehicle may further increase their in vivo delivery. The use of NPs as adjuvants holds promise in this regard due to the possibility of modifying their size, composition, degradation and release kinetics and their capability for efficient targeting of antigen presenting cells (APCs).
BCG vaccination fails to prevent adult tuberculosis. The present novel PCL system entrapping an antigen from the RD1 region of mycobacteria which is deleted in BCG may prove as an effective alternate vaccination strategy for the prevention of tuberculosis. The system is biocompatible, safe and will also be a cost effective subunit vaccine for mass immunization programs.
Conclusion
The 60nm PCL system we synthesized is perfectly biocompatible and biodegradable with slow antigen release kinetics. The particles cause enhanced Th1/Th2 response and CD8+ T cell response of the M.tb antigen, ESAT 6. Hence they can be safely used for developing an effective adjuvant for subunit vaccines against TB.
Acknowledgements
We thank ICMR for supporting a research fellow for the work. We thank Department of Biotechnology for funding the patent related expenses. We also thank the Electron Microscopy Facility at All India Institute of Medical Sciences, N. Delhi.
Abbreviations:
ESAT 6- early secreted antigenic target 6
References
- 1.W H Boom. (2007) New TB vaccines: is there a requirement for CD8 T cells?. , J Clin Invest 117, 2092-2094.
- 2.T H Ottenhoff, S H Kaufmann. (2012) Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog.
- 3.A S Mustafa. (2002) Development of new vaccines and diagnostic reagents against tuberculosis. , Mol Immunol 39, 113-119.
- 4.J Moon, Suh H, A V Li, C F Ockenhouse, Yadava A. (2012) Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci 109, 1080-1085.
- 5.Yue H, Wei W, Fan B, Yue Z, Wang L. (2012) The orchestration of cellular and humoral responses is facilitated by divergent intracellular antigen trafficking in nanoparticle-based therapeutic vaccine. , Pharmacol Res 65, 189-197.
- 6.N K Gupta, Tomar P, Sharma V, V K Dixit. (2011) Development and characterization of chitosan coated poly-(ɛ-caprolactone) nanoparticulate system for effective immunization against influenza. , Vaccine 29, 9026-9037.
- 7.Jewell C M, López S C, Irvine D J. (2011) In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci 108, 15745-15750.
- 8.Han R, Zhu J, Yang X, Xu H. (2011) Surface modification of poly(D,L-lactic-co-glycolic acid) nanoparticles with protamine enhanced cross-presentation of encapsulated ovalbumin by bone marrow-derived dendritic cells. , J Biomed Mater Res A 96, 142-149.
- 9.Murillo M, M J Grilló, Reñé J, C M Marín, Barberán M. (2001) A Brucella ovis antigenic complex bearing poly-epsilon-caprolactone microparticles confer protection against experimental brucellosis in mice. , Vaccine 19, 4099-4106.
- 10.Singh J, Pandit S, V W Bramwell, H O Alpar. (2006) Diphtheria toxoid loaded poly-(epsilon-caprolactone) nanoparticles as mucosal vaccine delivery systems. , Methods 38, 96-105.
- 11.Florindo H F, Pandit S, Lacerda L, L M Gonçalves, H O Alpar. (2009) The enhancement of the immune response against S. equi antigens through the intranasal administration of poly-epsilon-caprolactone-based nanoparticles. , Biomaterials 30, 879-891.
- 12.Tomar P, V S Karwasara, V K Dixit. (2011) Development characterizations and evaluation of Poly(-ε-caprolactone)-based microspheres for hepatitis B surface antigen delivery. , Pharm Dev Technol 16, 489-496.
Cited by (1)
- 1.Aminu Nafiu, Audu Momoh Mumuni, 2023, , , (), 37, 10.1016/B978-0-323-85656-0.00003-6
