Abstract
Introduction:
Aflatoxins are cytotoxic andserve as one of the key risk factors of hepatocellular carcinoma. Currently, plants and extract are widely used as potential scavenging substances for the detoxification of mycotoxins. Thus, this study aims to investigate the activity of the crude ethanolic leaves extract from Alchorneacordifolia in aflatoxicosis prevention.
Material and Methods:
The phytochemical screening was performed through qualitative analysis based on coloring and/or precipitation reactions. Groups of rats were treated daily with a mixture dose of aflatoxin B1 (AFB1) at 150 µg/kg and the crude extract of Alchorneacordifolia at doses of 50, 100, and 300 mg/kg for 21 days. The body weight, biochemical, and histological assessments were determined.
Results:
The phytochemical screening revealed the presence of polyphenols, flavonoids, sterols and terpenoids, quinoid compounds, tannins catechic and alkaloids. AFB1 treatmentcaused a significant increase of transaminases, urea, and creatinine abundances but reduced the rates of albumin and total proteins. Alchorneacordifolia administration alleviated biochemical parameters and body weight gain compared with the AFB1 group (p<0.05). The histological lesions of organs (liver and kidney) caused by AFB1 were significantly improved after administration of the extract at a dose of 300 mg/kg.
Conclusion:
This plant plays a beneficial role in AFB1-induced injury and may be used in the treatment of aflatoxicosis.
Author Contributions
Academic Editor: Kai Wang, Institute of Subtropical Agriculture & University of Chinese Academy of Sciences, China
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Adépo Aholia Jean- Baptiste, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Aflatoxin B1 (AFB1) is one of the principal food contaminants and is mainly produced by mold strains such as Aspergillus flavus and Aspergillus parasiticus. This mycotoxin is one of the key risk factors of hepatocellular carcinoma (HCC) in human being in sub-saharan Africa and in the Middle East 12, 34. Many other reported harmful effects of aflatoxins such as growth delay, immune abnormalities and hepatic affections 13, 42. These toxic and carcinogenic effects caused by aflatoxin B1 are due to the oxidization of this molecule in the liver by P450 cytochromes of the families of CYP1A2, CYPA6 and CYPA4. These enzymes convert AFB1 into its carcinogenic form: the AFB1-8,9-epoxyde which establishes covalent bonds with the DNA and serum albumin to respectively produce AFB1-N7-guanine and AFB1-Lysin adducts 11, 36. These enzymes can oxidize AFB1 in several other metabolites such as Aflatoxin M1 (AFM1) which is regarded as possible carcinogen for human being 18. Several studies showed the capacity of scavengers in the protection against carcinogenesis and other toxicities caused by aflatoxins administration in pre-pretreatment or simultaneously with the carcinogenic. Synthetic scavengers such as butylated hydroxyl toluene (BHT), butylated hydroxyl anisole (BHA) and the propyl gallate showed their capacity to inhibit carcinogenesis caused by AFB1. However, the toxicity of these synthetic scavengers is a stem for their use in aflatoxicosis prevention 7, 20, 41. The increasing sensitivity of consumers to residual pollution and the toxic effects of these products prompted researchers to find out alternatives. These last years, many studies were interested in natural scavengers for their capacity to reduce aflatoxins toxicity. faAlchorneacordifoliais a widespread plant and largely used in tropical Africa for the treatment of many ailments such as urinary infections, diarrhoea, anaemia and rheumatism 22, 30, 40. This plant also has antibacterial 4, 22, 27, 31, antifungal 27, antiparasitic 5, 39, anti-spasmodic 30, 40 and anti-inflammatory 25 properties. Some pharmacological activities of Alchorneacordifoliawere due to its strong scavenging potential 23. The phytochemical studies of Alchorneacordifolia leaves mostly showed the presence of phenolic compounds, Flavonoids, Alkaloids, Saponosides and tannins 24, 25, 33. The purpose of this present study is to evaluate the scavenging potential of Alchorneacordifoliain the body protection against aflatoxicosis.
Material and Methods
Chemicals:
Aflatoxins B1 (AFB1) was bought at Fermentek Ltd (Jerusalem, Israel) and the Butylated hydroxyanisole (BHA) at Santa Cruz Biotechnology (Delaware, Canada). Kits of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea and creatinine were obtained from Randox Laboratories (Antrim, UK). Albumin and total protein measuring kits were purchased from QCA, (AMPOSTA, Spain). All the other reagents used, were of analytical quality.
Plant Material
The leaves of Alchorneacordifoliawere collected in the suburbs of Abidjan in March 2016. They were identified at the National center of Floristic (CNF) of the University of Felix Houphouët Boigny (Abidjan, Cote d’Ivoire). The leaves were dried for two weeks at ambient temperature.
Preparation of the Crude Ethanolic Leaves Extract of Alchornea Cordifolia.
The dry leaves of Alchorneacordifoliawere ground using a grinder and 100 grams of the powder were macerated in one liter of ethanol (95 %) and shaken using a magnetic stirrer for 72 hours at ambient temperature. Then, the alcoholic solution was filtered twice on absorbent cotton then on a whatman filter paper. The filtrates obtained were put together and evaporated at reduced pressure at 45˚C using a rotary evaporator. The mixture was then dried in an oven at 45˚C for 72 hours. Finally a marrow black powder were obtained (13.33 g) representing the ethanolic leaves extract of Alchorneacordifolia(EELAc). Different concentrations of EELAcwere prepared on the spot during experiment by dissolving the powder obtained in an olive oil.
Phytochemical Screening
The different chemical analyses were carried out through a phytochemical screening. It is a qualitative analysis based on coloring and/or precipitation reactions. This analysis was performed on the ethanolic crude leaves extract of Alchornea cordifolia according to the methodology described by Houghton 17. Table 1 points out the various chemical groups investigated and the specific reagents used.
Table 1. Methods used for the phytochemical screening and administration of tested substancesAnimal Material
Experiments were carried on white albino male rats, Rattus norvegicus of Wistar strain. These adult rats were between 12 to 16 weeks of age with a mean body weight of 152 ±4,5 g. Animals were fed with pellets and water ad libitum. They were acclimatized in cages for two weeks for them to be accustomed to the experiment environment. All experiments were performed in accordance with the European guidelines 2010/63/EU related to animals care 8.
Products Administration
Six groups of six male rats were set out. AFB1 (150 µg/kg) and tested substances (A. cordifolia: 50, 100, 300 mg/kg and BHA; 50 mg/kg) were dissolved in olive oil and the mixture was administrated orally to animals at a rate of 0.5 ml for 100 g/kg Bw 28 according to the model of Table 1. The administrations are done five days per week for twenty one days with resting periods (Table 1). In this experiment, the BHA was used as a standard scavenger. Its effects were compared with those of Alchornea cordifolia.
Sampling and Dosage of Required Parameters
Biochemical Parameters
On completion of the experiment, blood samples were collected in rat orbital sinus after being anesthetized using ether diethyl. Blood samples were centrifuged at 4000 tours/min for 10 minutes. Collected serum was put in an aliquot and preserved at -20 C for analyses. AST, ALT, albumin, total proteins, urea and creatinine were dosed with a commercial kit according to the manufacturer recommendations. Parameters were evaluated using a biochemistry automate of the type A 15 bio-system (Barcelona, Spain).
Histological Evaluation
All the animals were sacrificed by an anesthesia (ether diethyl) at the end of the experiment, organs (liver, kidneys) were immediately taken from each animal and preserved in formalin at 10 %. Organ samples were then colored with hematoxylin and eosin and were observed through optical microscope.
Statistical Analysis
Statistical analysis and graphs were performed using the soft-ware Graph Pad Prism 5.01 (San Diego, CA, United States). The results are the arithmetic mean of individual values assigned to the standard error (mean ± SEM). The results were analyzed statistically by ANOVA 2 followed by multiple comparison tests with Tukey–Kramer method. The difference between groups was considered significant when a probability (p) was <0.05.
Results
Phytochemical Screening
From 100 g of Alchorneacordifolialeaves, we obtained 13.33 g of crude leaves extract of Alchorneacordifolia, representing a yield of 13.33 %. The phytochemical screening of the ethanolic leaves extract of Alchorneacordifoliashowed the presence of polyphenols, the flavonoids, sterols and terpenoids, quinoid compounds, catechic tannins and alkaloids (Table 2).
Table 2. Bioactive metabolites of the ethanolic leaves extract of Alchornea cordifolia (EELac).Effect of Aflatoxin B1 on Rats Treated with EELac Rats Body Weight Evolution During Experiment
Animals of group 1 treated with olive oil for 21 days, showed a significant (P < 0.05) body weight growth of 52.46 g at the end of experiment (Table 3). Rats from groups 2, 3 and 4 showed a body weight growth ranging from 10.5 g (group 2) to 25.3 g (group 4). These body weight growths, though positive, were significantly inferior to that of group 1 (Control). The body weight gain of rats from group 5 and 6 were respectively 45.16 and 47.6 g. The body weight growth were not significantly different between group 5 and 6 compared to group 1 (Control). The animals showed a positive body weight gain ranging from 10.5 to 52.46 g. The evolution of the body weight growth of group 5 is similar to those of groups 1 and 6 treated respectively with the olive oil (Control) and standard scavenger.
Table 3. Evolution of rats mean body weight during experiment| Animals body weight (g) | ||||||
| group1 | group 2 | group 3 | group 4 | group 5 | group 6 | |
| D0 | 148,06±2,3 | 154,26±4,2 | 158,36±1,3 | 149,14±2,4 | 137,44±2,9 | 155,94±2,3 |
| D 21 | 200,52±7,4a | 164,76±2,2b | 174,27±3,7b | 174,44±3,5c | 182,60±5,2a | 203,54±7,6a |
| Body weight Variation (g) | +52,46d | +10,5e | +15,91e | +25,3f | +45,16d | +47,6d |
Effect of the Ethanolic Extract of AlchorneaCordifoliaon the Biochemical Parameters.
Effect of the Extract on Hepatic Enzymes.
The treatment of rats with the various substances caused a significant increase (P < 0.05) of transaminases rates in group 2 and 3 compared to the control (group 1). This increase was not dose dependent and varied from 262.4 ± 8.1 UI/L (group 2) to 257.5±9,2 UI/L (group 3) for ALT and from 274.16±7,ÚI / L (group 2) to 282.5±3,1UI / L (group 3) for AST (Table 4). In contrast, there is no significant difference between these rates for group 4, 5 and 6 compared to the control (group1). These rates were 115.4±7.7 UI/L (group 4), 100.22±2.3 UI/L (group 5) and 90.75±2.4 UI/L (group 6) for ALT and 203.2±4.9 UI/L (group 4), 180.13±5.5 UI/L (group 5) and 186.25±6.7 UI/L (group 6) for AST.
Table 4. Effect of the ethanolic extract of Alchornea cordifolia on the biochemical blood parameters| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
| ALT (UI/l) | 77,67±3,2a | 262,4±8,1k | 257,5±9,2k | 115,4±7,7a | 100,22±2,3a | 90,75±2,4a |
| AST (UI/l) | 170,03±5,1b | 274,16±7,3v | 282,5±3,1v | 203,2±4,9b | 180,13±5,5b | 186,25±6,7b |
| Creatinine (mg/l) | 5,43±0,1c | 9,62±1,2h | 10,74±1,3h | 7,96±1,3h | 5,81±1,2c | 6,17±1,1c |
| Urea (g/l) | 0,31±0,03d | 0,76±0,05z | 0,69±0,01z | 0,58±0,02z | 0,33±0,03d | 0,29±0,02d |
| Albumin (g/l) | 34,67±2,2 e | 19,5±2,5u | 21,75±1,4u | 23±1,1u | 32,18±2,2e | 31,5±1,3e |
| Total proteins (g/l) | 60,67±4,1f | 34,26±2,1r | 36,75±3,1r | 41,82±3,4r | 63,98±4,5f | 67,3±2,6f |
Effect of the Extract on Renal Function
Substances administration caused a significant increase of creatinine, urea rate and a reduction of albumin and total proteins rates for group 2, 3 and 4 compared to the control. These values increase from 7.96±1.3 mg/l (group 4) to 10.74 ±1.3 mg/l (group 3) for creatinine and from 0.58 ± 0.02 g/l (group 4) to 0.76 ± 0.05 g/l (group 2) for urea and decrease from 23 ± 1.1g/l (group 4) to 19.5± 2.5 g/l for albumin and from 41.82 ±3.4 g/l to 34.26 ± 2.1(g/l) for total proteins. The evolution of these biochemical parameters is not statistically significant between groups 5 and 6 compared to the control (P < 0.05) (Table 4). The results of the biochemical parameters showed that the evolution of transaminases, urea, albumin and total proteins in group 5 was similar to that of group 6 which received the standard scavenger (BHA). There was no significant difference between the values of these parameters in groups 5 and 6 compared to the control group (group 1) at P < 0.05.
Results of the Histopathological Evaluations Hepatic Tissues
The administration of AFB1 alone caused a sinusoid capillary dilation with hemorrhagic contents (Figure 1 A). In contrast, the administration of the mixture AFB1+EELac at a dose of 300 mg/kg (group 5) or AFB1+ BHA at a dose of 50 mg/kg (group 6) caused the disappearance of these histological abnormalities of the liver (Figure 1 B, C).
Figure 1.Effect of AFB1 on liver tissues. A photomicrograph of liver section from (A) Liver of rat treated with AFB1 alone (group 2) showing a sinusoid capillary dilation with hemorrhagic contents (arrow), (B) rat treated with AFB1 + EELac at 300 mg/kg (group 5) showed the normal hepatocytes (arrow) ¶ and portal area (star) without histological abnormalities¶, (C) rat Treated with AFB1+ BHA at 50 mg/kg (group 6) showed the normal hepatocytes (arrow) ¶ and portal area (star) without histological abnormalities.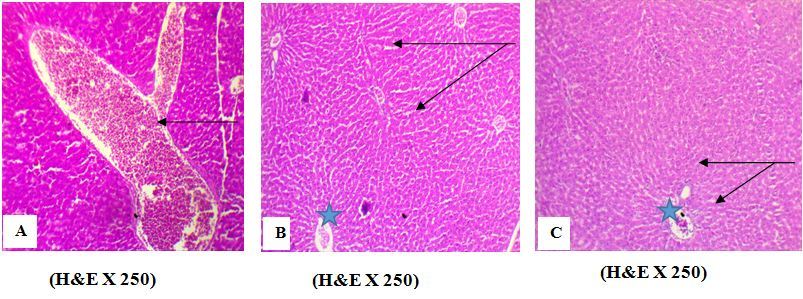
Figure 2.Effect of AFB1 on kidney tissues. A photomicrograph of kidney section from (D) kidney of rat treated with AFB1 alone (group 2) showed an atrophy of renal tubules (arrow), (B) Rat Treated with AFB1 + EELac at 300 mg/kg (group 5) showed a normal histology of renal tubules (arrow), (C) Rats Treated with AFB1 + BHA at 50 mg/kg (group 6) showed a normal histology of renal tubules and renal glomeruli (arrow). ¶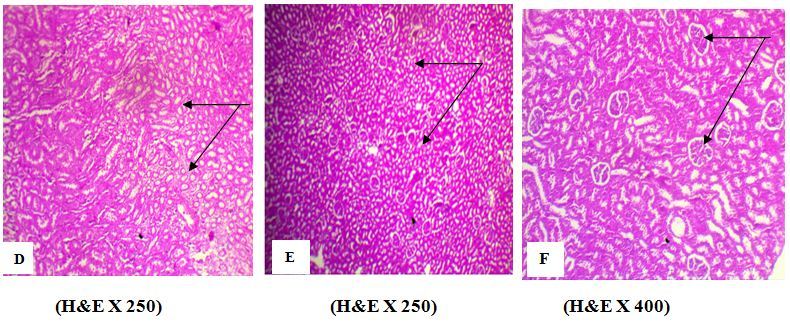
Renal Tissues
The histopathological examinations of the kidney showed that AFB1 caused an atrophy of renal tubes (Figure 2 D). However, the administration of the mixture AFB1+EELac at a dose of 300 mg/kg (group 5) or AFB1+ BHA at a dose of 50 mg/kg (group 6) caused the disappearance of these histological abnormalities observed in the renal tubes (Figure 2 E, F).
Discussions
Extraction and Phytochemical Screening
Many methods were developed to fight aflatoxicosis. Several compounds such as food, scavengers, plants, pharmaceutical products are used in various experimental studies to modulate the damage entailed by AFB1 on some organs (liver, kidney, lungs) and macromolecules (DNA, proteins) 10, 14, 16. Hepatocellular carcinoma induced by AFB1 can be modified in animals by chemo-protective substances such as phenolic antioxidants (butylated hydroxy anisole, ethoxyquine) 21, 43. In this study, the effect of the crude ethanolic leaves extract of Alchorneacordifolia(EELac) was investigated on aflatoxicosis. The choice of Alchorneacordifoliawas made according to an ethnobotanical survey which demonstrates that, this plant is widely used for the treatment of several pathologies linked to oxydative stress such as diabetes, high blood pressure and hemorrhoids. Moreover, this plant can be easily found in Côte.d'Ivoire. The ethanolic extraction of Alchorneacordifolia leaves gave a yield of 13.33 %. This rate was close to that reported by Okwu 32 which was 10.06 %. The phytochemical screening of Alchorneacordifolia leaves revealed the presence of several secondary metabolites such as phenolic compounds, flavonoids, sterols and terpenoids, quinoid compounds, tannins and alkaloids. These results are in agreement with those of several authors who observed the presence of these compounds in the leaves extract of this plant 24, 29, 33. Studies also reported that flavonoids and polyphenols respectively account for 14.4% and 42.2 % of dry matter of Alchorneacordifolia leaves 24.
Body Weight Evolution
In order to evaluate the efficacy of Alchorneacordifoliaagainst aflatoxicosis, we prepared increasing doses of 50, 100 and 300 mg/kg of body weight of the ethanolic leaves extract of this plant. The choice of these doses were carried out from preliminary studies. The BHA is a synthetic antioxidant authorized as food additive. We used it as a standard antioxidant in this study at a dose of 50 mg/kg according to the studies of Shatat 37. Rats treated with AFB1 at a dose of 150 µg/kg for three weeks showed a weak increase in animals body weight (10.5 g) compared to the initial weight at D0. This growth though positive, is significantly lower than that of the animals of the control group which received the olive oil. This variation of body weight is due to the toxicity of aflatoxin B1 administered at a dose of 150 mg/kg. Groups 3, 4 and 5 which received the mixture containing aflatoxin at a dose of 150 mg/kg and the extract at doses ranging from 50 to 300 mg/kg also showed a positive body weight gain. The recorded body weight gain for these three groups vary from 15.91 g to 45.16 g. These body weight gain are dose-dependent. Therefore, the mixture having the highest concentration of EELac (300mg/ kg) showed a body weight gain of 45.16 g which is significantly different from the body weight gain of group 2 (treated only with aflatoxin B1) but close to the values of control groups (group 1 and 6). Thus, by increasing the extract concentration in EELac in the mixture, we gradually restore animals’ normal growth. These results are similar to those of several authors who reported that the acute or subacute forms of aflatoxicosis are dominated by anorexia, depression and body weight loss 15, 16. These observed effects are due to the inhibition of proteins synthesis and DNA. Several authors reported that body weight decrease following an intake of AFB1 is linked to a reduction of food consumption causing a protein catabolism 38.
Effect of AFB1 on Proteins, Hepatic Enzymes and Renal Function in the Presence of EELac
Aflatoxins are hepatotoxic for all vertebrates and induce a degeneration, hepatocytes necrosis and modify the liver function 35. Administration of AFB1 at a dose of 150µg/kg for three weeks caused a significant increase of ALT and AST rates. This significant rise of transaminases rate is caused by the toxic effects of AFB1 in the hepatic tissue and the biliary system 1. These results are in agreement with those reported in literature indicating that, liver is the principal target of aflatoxins 2, 9. The results of the biochemical analyses are in accordance with the histological examinations which showed lesions in the hepatic tissues. The rise in the rate of transaminases is due to the sinusoid capillary dilation with hemorrhagic contents revealed by liver tissues through histological examinations. These cytoplasmic enzymes were released in plasma after the lesions of hepatic tissue lesions. Similar effects on liver tissues were reported by several studies in animals receiving aflatoxin B1 2, 3, 19. The administration of AFB1 caused a rise in the rates of urea and creatinine. The rise in the rate of these products is a sign of renal dysfunction due to an atrophy of renal tubes and glomeruli. AFB1 also caused a reduction of albumin and total proteins rates in serum, this is due to the reduction of proteins synthesis in the liver and to the production of RNA 1. These results are in agreement with those reported in literature and clearly showed the toxic effects of AFB1 on proteins and on hepatic and renal tissues 1. The rates of transaminases, urea, creatinine, albumin and total proteins gradually came to normalcy by the administration of a mixture of AFB1 and the extract of Alchorneacordifolia. These results are corroborated by those of Mohammed 26 who reported that Alchornea cordifoliaacts on liver by restoring the rate in serum of ASAT and ALAT in diabetic rats. The effects of EEFac at a dose of 300 mg/kg are similar to those of BHA at a dose of 50 mg/kg. EEFac could protect the body organs and tissues against AFB1 toxicity following the same mechanism as BHA. Indeed, BHA is a standard phenolic antioxidant which acts effectively against the cancerogenicity of AFB1 by impeding its oxydative way of biotransformation in the liver 6. Similar effects of EEFac to those of BHA could be essentially due to its phenolic compounds and the flavonoids revealed by the chemical screening.
Conclusions
Aflatoxins are a real issue of public health for African countries. The implication of this mycotoxin in the appearance of many pathologies such as hepatocellular carcinoma requires urgent and adequate prevention measurements. The pharmacological effect of the ethanolic leaves extract of Alchorneacordifoliain the reduction of aflatoxicosis was investigated. This study showed that, this plant has a protective effect on some body organs and tissues such as the liver and the kidneys against the toxicity of aflatoxin B1. These pharmacological properties of Alchorneacordifoliaare due to flavonoids and to the phenolic compounds. The effect of this plant which is dose-dependent can be used in the prevention of some pathologies linked to the oxidative stress. In addition deep studies should be undertaken in order to evaluate the efficacy of this plant in the protection of other body organs on which aflatoxins have a toxic effect.
References
- 1.M A Abdel-wahhab, S A Nada, F A Khalil. (2002) Physiological and toxicological resposes in rats fed aflatoxin-contaminated diet with or without sorbent materials. Anim Feed Sci. , Tech 97, 209-219.
- 2.Abdel-Wahhab M A, Hassan N S, El-Kady A A, Khadrawy Y A, El-Nekeety A A. (2010) Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. , Food and Chemical Toxicology 48, 733-742.
- 3.M A Abdel-Wahhab, S A Nada, I M Farag, N F Abbas, H A Amra. (1998) Potential protective effect of HSCAS and bentonite against dietary aflatoxicosis in rat with special reference to chromosomal aberrations. , Natural Toxins 6, 211-218.
- 4.Ajali U. (2000) Antibacterial activity of Alchornea cordifolia stem bark. , Fitoterapia 71, 436-438.
- 5.Banzouzi J T, Prado R, Menan H, Valentin A, Roumestan C. (2002) In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. , Journal of Ethnopharmacology 81, 399-401.
- 6.Ch'ih J J, Biedrzycka D W, Lin T, Khoo M O, Devlin T M. (1989) 2(3)-tert-butyl-4-hydroxyanisole inhibits oxidative metabolism of aflatoxin B1 in isolated rat hepatocytes. , Proc. Soc. Exp. Biol. Med 192, 35-42.
- 7.Chen C, A M Pearson, J I Gray. (1992) Effects of synthetic antioxidants (BHA, BHT and PG) on the mutagenicity of IQ-like compounds. , Food Chem 43, 177-183.
- 8.EU Directive 2010/63/EU of the Europe an Parliament and of the Council of22September2010 on the protection of animals used for scientific purposes. Official Journal of the EuropeanUnionL276/33 , http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm; .
- 9.J H Do, D K Choi. (2007) Aflatoxins: detection, toxicity, and biosynthesis. , Biotechnol. Bioprocess Eng 12, 585-593.
- 10.J A Elegbede, M N Gould. (2002) Monoterpenes reduced adducts formation in rats exposed to aflatoxin B1. , African Journal of Biotechnology 1, 46-49.
- 11.J M Essigmann, R G Croy, A M Nadzan, Busby W F, V N Reinhold. (1977) Structural identification of the major DNA adduct formed by aflatoxin b1 in vitro. , Proc. Natl. Acad. Sci. USA 74, 1870-1874.
- 12.Ferlay J, H R Shin, Bray F, Forman D, Mathers C. (2010) Estimates of worldwide burden of cancer in 2008: GLOB OCAN. , Int. J. Cancer 127, 2893-917.
- 13.Gong Y Y, Egal S, Hounsa A, Turner P C, Hall A J. (2003) Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: The critical role of weaning. , International Journal of Epidemiology 32, 556-562.
- 14.Gradelet S, Le Bon AM, Berges R, Suschetet M, Astorg P. (1998) Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: Role of the modulation of aflatoxin B1 metabolism. , Carcinogenesis 19, 403-411.
- 15.Guerre P, Galtier P, Burgat V. (1996) Les aflatoxicoses chez l'animal : des manifestations cliniques aux mécanismes d'action. Revue Méd. , Vét 147, 497-51.
- 16.Hamzawy M A, EL-Denshary E S, Hassan N S, Manaa F, Abdel-Wahhab M A. (2012) Antioxidant and hepatorenoprotective effects of thyme vulgaris extract in rats during aflatoxicosis. , Global Journal of pharmacology 6, 106-117.
- 17.P J Houghton, Raman A. (1998) Laboratory handbook for the fractionation of natural extracts, New York,Ed.Chapman and Hall p.208.
- 18.Aflatoxins IARC. (2002) . In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans;IARC Press:Lyon,France 62, 171-300.
- 19.Imaoka S, Ikemoto S, Shimada T, Funae Y. (1992) Mutagenic activation of aflatoxin B1 by pulmonary, renal, and hepatic cytochrome P450s from rats. , Mutation Research 269, 231-236.
- 20.Ito N, Fukushima S, Hagiwara A, Shibata M, Ogiso T. (1983) Carcinogenicity of butylated hydroxyanisole in F344 rats. , J. Natl. Cancer Inst 70, 343-352.
- 21.Jhee E C, Ho L L, Lotlikar P D. (1988) Effect of Butylated Hydroxyanisole Pretreatment on in vitro hepatic aflatoxin B1-DNA binding and aflatoxin B1-glutathione conjugation in rats. , Cancer Res 48, 2688-2692.
- 22.Kambu K, Tona L, Kaba S, Cimanga K, Mukala N. (1990) Antispasmodic activity of extracts proceeding of plant, antidiarrhoeic traditional preparations used in Kinshasa. , Zaire. Ann. Pharm. FR 48, 200-208.
- 23.Kouakou–Siransy G, Sahpaz S, Irié‐Nguessan G A, Dattes Y J, Kablan J.(2010a) Effetcs of Alchornea cordifolia on elastase and superoxide anion produced by human neutrophils. , Pharmaceutical Biology 48, 128-133.
- 24.Kouakou-Siransy G, Sahpaz S, Irié-Nguessan G, Y J Datte, Kablan J. (2010) Oxygen species scavenger activities and phenolic contents of four West African plants. , Food Chemistry 118, 430-435.
- 25.Mavar-Manga H, Haddad M, Pieters L, Baccelio C, Penge A. (2008) Antiinflammatory compounds from leaves and root bark of Alchornea cordifolia. , (Schumach and Thonn.) Mull. Arg. J Ethnopharmacol 115, 25-29.
- 26.R K Mohammed, Ibrahim S, S E Atawodi, E D, J B Suleiman. (2012) The Study of the effects of n-butanol fraction of Alchornea cordifolia leaf extract on lipid profile and liver enzymes in streptozotocin-induced diabetic rats. , Glob. J. Med. Plant. Res 1, 1-7.
- 27.Muanza D N, Kim B W, Euter K L, Williams L. (1994) Antibacterial and antifungal activities of nine medicinal plants from Zaire. , Int J Pharmacog 32, 337-345.
- 28.Neeta M, Ramtej J V. (2007) Ameliorative effect of curcumin on aflatoxin-induced toxicity in DNA, RNA and protein in liver and kidney of mice. , Acta Poloniae Pharmaceutica-Drug Research 64, 497-502.
- 29.A H N’Guessan, C E Déliko, J A Mamyrbékova-Békro, Y A Békro. (2011) Teneurs en composés phénoliques de 10 plantes médicinales employées dans la tradithérapie de l’hypertension artérielle, une pathologie émergente en Côte d’Ivoire. Revue de génie industriel 6. 55-61.
- 30.F O Ogungbamila, Samuelsson G. (1990) Smooth muscle relaxing flavonoids from Alchornea cordifolia (leaves). , Acta Pharmaceutica Nordica 2, 421-422.
- 31.I N keke, A O Ogundaini, F O Ogungbamila, Lamikanra A. (1999) Antimicrobial spectrum of Alchornea cordifolia leaf extract. , Phytotherapy Research 13, 67-69.
- 32.D E Okwu, N D Ukanwa. (2010) Isolation, characterization and antibacterial activity screening of anthocyanidine glycosides from Alchornea cordifolia (Schumach. , and Thonn.) Mull. Arg. leaves. E-Journal of Chemistry 7, 41-48.
- 33.Osadebe P, Okoye F, Uzor P, Nnamani N, Adiele I. (2012) Phytochemical analysis, hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. , Asian Pac. J. Trop. Med 5, 289-93.
- 34.Parkin D M, Bray F I, Devesa S S. (2001) Cancer burden in the year 2000. The global picture. , Eur. J. Cancer 37, 4-66.
- 35.Riley R T, Pestka J. (2005) Mycotoxins: Metabolism, mechanism, and biochemical markers. In: The Mycotoxin. Blue Book, Diaz D.E. (ed.) , Nottingham 279-294.
- 36.Sabbioni G, P L Skipper, Buchi G, S R Tannenbaum. (1987) Isolation and characterization of the major serum albumin adduct formed by aflatoxin b1 in vivo in rats. , Carcinogenesis 8, 819-824.
- 37.A R Shatat, H M Eldien, M Y Nassar, A O Mohamed, A H Hussein. (2013) Protective Effects of Copper (I)-Nicotinate Complex against Aflatoxicosis. , The Open Toxicology Journal 6, 1-10.
- 38.E N Tessari, C A Oliveira, A L Cardoso, D R Ledoux, G E Rottinghaus. (2006) Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. , Br. Poult. Sci 47, 357-364.
- 39.Tona L, Kambu K, Ngimbi N, Cimanga K, A J Vlietinck. (1998) Antiamoebic and phytochemical screening of some Congolese medicinal plants. , Journal of Ethnopharmacology 61, 57-65.
- 40.Tona L, Kambu K, Ngimbi N, Mesia K, Penge O. (2000) Antiamoebic and spasmolitic activities of extracts from some antidiarrhoeal traditional preparations used in Kinshasa. , Congo. Phytomedicine 7, 31-38.
- 41.A V Tran. (2013) . Do BHA and BHT Induce Morphological Changes and DNA Double-Strand Breaks in Schizosaccharomyces pombe? Scripps Senior Theses, Paper 152 .
Cited by (1)
- 1.Marin Daniela E., Taranu Ionelia, 2023, , , (), 351, 10.1007/978-3-031-42855-5_13

