Abstract
A bioassay-guided fractionation of petroleum ether, EtOAc and n-BuOH soluble parts of the 80% hydromethanol extract was performed to investigate the antioxidant activity of Secamoneafzeliiaerial parts using DPPH free radical scavenging assay. The results revealed that EtOAc and n-BuOH soluble parts have moderate to good DPPH radical scavenging activity (EC50 = 139.3 and 30.5 μg/mL, respectively). Therefore, from the most active fractions of EtOAc and n-BuOH soluble parts were isolated two new flavonoid diglycosides quercetin-3-O-β-d-apiofuranosyl-(1→2)-α-l-rhamnopyranoside and genkwanin-8-C-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside in addition to nine known compounds (2-10). Their structures were elucidated on the basis of spectroscopic data including 1D- and 2D-NMR and ESI-MS. The ability of the isolated compounds to scavenge the DPPH was evaluated. The new compound 1, quercitrin (3) and rutin (6) have antioxidant potential with EC50 values ranging from 8.4 to 13.6 µg/mL, compared to the standard ascorbic acid (EC50 7.4 µg/mL).
Author Contributions
Academic Editor: Zhiguo Zhou, Luna Innovations Inc.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Abdulmagid Alabdul Magid, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Antioxidants are compounds that inhibit or delay the oxidation process by blocking the initiation or propagation of oxidizing chain reaction due to oxidative stress 1. Antioxidants were known as free radical scavengers, reducing agents, chelators of pro-oxidant metals, or as quenchers of singlet oxygen. Described as an imbalance between free radicals and the body’s ability to detoxify these molecules or repair the resulting damage, oxidative stress causes extensive damage to biological molecules such as DNA, lipids and proteins 2, 3. Thus, it is the cause of several diseases including cancer, cataracts, amyotrophic lateral sclerosis, accelerated aging, the acute respiratory distress syndrome and pulmonary oedema 4, 5. In order to provide a solution to this problem, we propose to contribute to the search for new antioxidants from indigenous natural sources 6, 7, 8. In our continued search for new bioactive compounds from Ivory Coast medicinal plants 8, we investigated Secamoneafzelii (Roem. and Schult.) K. Schum, a creeping woody climber belonging to the family Apocynaceae 9. S. afzeliiis used in traditional medicine for stomach problems, diabetes, colic, dysentery and also for kidney problems 10. The decoction of the entire plant is prescribed for cough, catarrhal conditions and as galactogogue. For the treatment of gonorrhoea, the whole plant is crushed with fresh palm nuts and oil 9. Previous studies have shown that S. afzelli has antioxidant, anti-inflammatory and antimicrobial properties 11, 12, 13. A phytochemical screening of the methanol extract of the leaves and the stems of S. afzeliishowed the presence of high amount of flavonoids, but none, to the best of our knowledge, have reported their structural elucidation 14. Lack of chemical scientific data about the flavonoids contents of S. afzelii and the antioxidant property of this plant, prompted us to make this study. A bioassay-guided fractionation of the hydromethanol extract using DPPH free radical scavenging assay was used to investigate the antioxidant activity and isolate flavonoids from the most active fractions.
Results and Discussion
The 80% hydromethanol extract of the aerial parts of S. afzeliiwas concentrated and partitioned successively with petroleum ether (PE), ethyl acetate (EtOAc) and n-butanol (n-BuOH), followed by concentrating. The crude hydromethanol extract, the PE soluble part, EtOAc soluble part and n-BuOH soluble part were tested for their radical scavenging activity by DPPH assay. The results indicated that the n-BuOH soluble part has significant activity with IC50 value of 30.5 μg/mL, whereas the hydromethanol extract and EtOAc soluble part exhibited a moderate activity with IC50 value of 150.5 and 139.3 μg/mL, respectively. In order to isolate the compounds involved in this antioxidant action, a bioassay-guided fractionation strategy was applied throughout the separation procedure.
Four fractions (from B1 to B4) were obtained from the n-BuOH soluble part by vacuum liquid chromatography (VLC) over RP-18. The fraction B2 exhibited the highest DPPH radical scavenging activity with an EC50 of 30.3 μg/mL (Table 1). Accordingly, fraction B2 was subjected to column chromatography (CC) fractionation over silica gel and the resulting subfractions were tested for their DPPH radical scavenging activity. The active subfractions were further purified by semi-preparative HPLC affording two new compounds (1 and 11) in addition to kaempferol-3-O-β-d-apiofuranosyl-(1→2)-α-l-rhamnopyranoside (2) 15, rutin (6) 16, myricetin 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside (7) 17, kaempferol-3-O-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside (8) 18, mauritianin (9)19, and vicenin-2 (10) 20 (Figure 1).
Table 1. DPPH radical scavenging activities of 80% MeOH extract, AcOEt and n-BuOH soluble parts, fractions, and isolated compounds from Secamone afzelii.| EC 50 ± S.D. (µg/mL) | EC 50 ± S.D. (µg/mL) | ||
| 80% MeOH extract | 150.5 ± 7 | ||
| AcOEt extract | 139.3 ± 8 | n-BuOH extract | 30.5 ± 1.2 |
| A1 | > 200a | B1 | > 200a |
| A2 | 66.5 ± 1.4 | B2 | 30.3 ± 0.5 |
| A3 | 50.3 ± 1.5 | B3 | 90.2 ± 1.5 |
| A4 | > 200a | B4 | > 200a |
| Compounds isolated from the fraction A3 | Compounds isolated from the fraction B2 | ||
| 3 | 13.6 ± 0.5 | 1 | 12.5 ± 0.8 |
| 4 | > 200a | 2 | 59 ± 1.2 |
| 5 | 44.3 ± 1.1 | 6 | 8.1± 0.2 |
| 7 | 31 ± 0.4 | ||
| 8 | > 200a | ||
| 9 | > 200a | ||
| 10 | > 200a | ||
| Ascorbic acid b | 7.4 ± 0.2 | 11 | > 200a |
Figure 1.Chemical structures of flavonoids 1- 11, isolated from Secamone afzelii.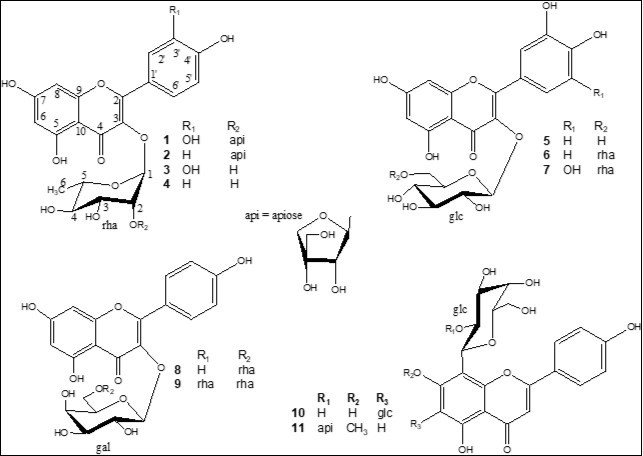
The EtOAcfraction was also fractionated by VLC over RP-18 affording fractions A1-A4. The most active fraction (A3) was subjected to VLC over silica gel and the active fractions were purified by semi-preparative HPLC affording quercitrin (3) 21, afzelin (4) 21, and isoquercitrin (5) 22 (Figure 1). The structural assignments of these compounds were made by HR-ESI-MS, 1D and 2D-NMR analysis.
Compound 1, (α)d20 -50.2, was isolated as a yellow amorphous powder. The positive HR-ESI-MS showed a molecular ion peak at m/z 603.1329 (M + Na)+ (calcd for C26H28O15Na, 603.1323) enabling us to determine the molecular formula C26H28O15. The UV spectrum displayed maximum absorption bands of a flavonol skeleton at lmax 257 and 357 nm 23. The 1H and 13C NMR spectra of 1 comprised resonances corresponding to aromatic and glycosidic protons and carbons. The A-ring of the flavonol was represented by two meta-coupled resonances at δH 6.23 (d, J = 2.1 Hz, δC 99.8) and δH 6.39 (d, J = 2.1 Hz, δC 94.8), assigned to H-6 and H-8, respectively (Table 2). The 1H and COSY NMR spectra of 1 exhibited in aglycone region an ABX system at δ 7.35 (1H, d, J= 2.1 Hz, H-2'), 7.32 (1H, dd, J= 8.3 and 2.1 Hz, H-6') and 6.95 (1H, d, J= 8.3 Hz, H-5'), due to a 3',4'-disubstituted B-ring. Complete assignment of the remaining resonances of the aglycone in the 13C NMR spectrum of 1 was achieved by analysis of the HSQC and HMBC data, which confirmed the presence of quercetin (3,5,7,3',4'-pentahydroxy-flavone). A full list of the corresponding assignments is given in Table 217. The sugar part of 1 consisted of two residues as evidenced by 1H and 13C NMR spectra which displayed two anomeric protons at δH 5.43 (J = 1.6 Hz) and 5.15 (J = 2.6 Hz), whose carbon resonances were assigned by HSQC-Jmod experiments at δC 102.1 and 112.2, respectively. The first sugar unit with its anomeric proton resonating at δH 5.43 was identified as α-l-rhamnopyranose (rha), characterized by the small coupling constants JH-1,H-2 (1.6 Hz) and its methyl group at δH-6 1.10 (d, J = 6.2 Hz) and δC-6 18.0 as summarized in Table 2. The second sugar residue showed NMR signals for two methines and two methylenes groups, in addition to a quaternary carbon, in agreement with an apiofuranose moiety 24. The deshielded chemical shifts of the anomeric signals at δH 5.15 (d, J = 2.7 Hz) and δC 112.1 and the HMBC correlations observed between anomeric proton H-1 with its C-3 and C-4 indicate that this sugar residue is a β-erythroapiofuranose (api). The most commonly d- configuration for apiofuranose and l- for rhamnopyranose were assumed on the basis of the result of the acid hydrolysis of flavonoids mixture. A correlation between H-1-rha and δC 136.2 in the HMBC spectrum of 1 defined C-3 of quercetin as the site of O-glycosylation, whereas the correlations from H-1-api to the downfield-shifted C-2-rha (δC 79.4) indicated that the api residue was 2-O-linked to rha. Therefore, the structure of compound 1 was determined as quercetin-3-O-β-d-apiofuranosyl-(1→2)-α-l-rhamnopyranoside.
Table 2. 1H and 13C NMR spectroscopic data for compounds 1 and 11 (in CD3OD) isolated from Secamone afzelii.| 1 | 11 | |||
| δH m (J in Hz) | δ C | δH m (J in Hz) | δ C | |
| Aglycone | ||||
| 2 | - | 159.4 | - | 167.0 |
| 3 | - | 136.2 | 6.64 s | 103.6 |
| 4 | - | 179.6 | - | 184.4 |
| 5 | - | 163.0 | - | 163.8 |
| 6 | 6.23 d (2.1) | 99.8 | 6.62 s | 96.0 |
| 7 | - | 166.0 | - | 165.1 |
| 8 | 6.39 d (2.1) | 94.8 | - | 106.6 |
| 9 | - | 158.4 | - | 157.7 |
| 10 | - | 106.0 | - | 106.0 |
| 1 | - | 123.0 | - | 123.6 |
| 2 | 7.35 d (2.1) | 116.9 | 8.03 d (8.4) | 130.2 |
| 3 | - | 146.4 | 6.98 d (8.4) | 116.5 |
| 4 | - | 149.2 | 162.9 | |
| 5 | 6.95 d (8.3) | 116.4 | 6.98 d (8.4) | 116.5 |
| 6 | 7.32 dd (8.3, 2.1) | 122.6 | 8.03 d (8.4) | 130.2 |
| OCH3 | 3.96 s | 57.0 | ||
| 3-O-rha | 8-C-glc | |||
| 1 | 5.43 d (1.6) | 102.1 | 5.02 d (9.2) | 73.5 |
| 2 | 4.21 dd (3.5, 1.6) | 79.4 | 4.25 t (9.2) | 77.4 |
| 3 | 3.89 dd (9.7, 3.5) | 71.7 | 3.64 t (9.2) | 80.9 |
| 4 | 3.34 m | 73.6 | 3.67 t (9.2) | 72.2 |
| 5 | 3.59 m | 71.9 | 3.44 m | 83.0 |
| 6 | 1.10 d (6.2) | 18.0 | 3.84 m | 63.0 |
| 3.94 dd (11.7-3.9) | ||||
| 2rha-O-api | 2glc-O-api | |||
| 1 | 5.15 d (2.6) | 112.2 | 5.14 br s | 111.1 |
| 2 | 3.95 d (2.6) | 77.9 | 3.77 br s | 77.8 |
| 3 | 80.4 | 80.9 | ||
| 4a | 3.72 d (9.8) | 75.0 | 2.53 d (9.8) | 74.2 |
| 4b | 3.85 d (9.8) | 3.13 d (9.8) | ||
| 5 | 3.57 s | 65.5 | 3.30 s | 65.4 |
Compound 11 was obtained as yellow powder and exhibited UV absorptions at 262 and 336 nm characteristic of a flavone 23. The HR-ESI-MS spectrum displayed a molecular ion peak (M + Na)+ at m/z 601.1528, (calcd for C27H30O14Na, 601.1533) in agreement with a molecular formula of C27H30O14. The 1H and 13C NMR spectra of 11 comprised resonances corresponding to aromatic and glycosidic protons and carbons, and a methoxy group (δC 57.0; δH 3.96). The 1H NMR spectrum of 11 (Table 2) indicated signals of two set of doublets at δ 8.03 (2H, d, J = 8.4 Hz) and 6.98 (2H, d, J = 8.4 Hz), due to the protons H-2′, 6′ and H-3′, 5′ of a 4′-hydroxyphenyl moiety in B-ring, and two singlets at δ 6.64 and 6.62, due to the protons at C-3 and C-6 in rings C and A of a flavone skeleton, respectively. Full identification of the aglycone was finally achieved by 2D-NMR spectroscopy. The HMBC correlation between δH 3.96 (OCH3) and δc 165.1 (C-7) placed the OCH3 at C-7 of the aglycone, which led to the genkwanin structure (7-methoxy-3,5,4'-trihydroxy-flavone) 25. The HMBC cross peaks from H-3 (δH 6.64) to C-1' (δC 123.6), C-2 (δC 167.0), C-4 (δC 184.4), and C-10 (δC 106.0), and from H-6 (δH 6.62) to C-5 (δC 163.8), C-7 (δC 165.1), C-8 (δC 106.6), and C-10 confirmed that the two singlet protons were at C-3 and C-6, respectively. Two anomeric proton resonances corresponding to an O-linked and a C-linked sugars were present in the 1H NMR spectrum at δH5.02 (1H, d, J=9.2 Hz, δC73.5) and 5.14 (1H, br s, δC111.1). Based on the results of the acid hydrolysis of flavonoids mixture, the chemical shift values, multiplicities and J-values, the magnitudes of their J1,2 coupling constants and the analysis of 2D NMR data, the two sugar residues were elucidated as a β-d-glucopyranose (glc) (δH-1 5.02), and a β-d-apiofuranose (api) (δH-1 5.14). The glycosylation position was unambiguously determined at the C-8 position by the appearance of cross-peaks of the glucosyl anomeric proton H-1 (δ 5.02, d, J = 9.2 Hz) with the carbon signals at δ 106.6 (C-8), 165.1 (C-7), and 157.7 (C-9) in the HMBC spectrum. From these data, the sugar substituent at C-8 of the aglycone moiety gave a pattern of 13C NMR signals similar to those in precatorin III, in which the disaccharide chain api-(1→2)-glc- was C-linked at the C-6 position of the genkwanin 26. The signals of glc-C-1 (δ 73.5) and glc-C-2 (δ 77.4) of the sugar moiety showed dowfield shifts of 2.4 and 3.2 ppm, compared with the corresponding data (δ 71.1, 74.2) of precatorin III, whereas the signals of glc-C-1 and glc-C-2 where identical to those of ficuflavoside in which the same disaccharide chain was C-linked to the C-6 position of the apigenin 17. The comparison of the 13C NMR signals of 8-C-glucoside genkwanin 27 and 6-C-glucoside genkwanin 26 confirmed the position of C-glycosylation of 11. On the basis of the above evidence, the structure of 11 was established as genkwanin-8-C-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside.
The DPPH radical scavenging activity of compounds 1-11 was measured (Table 1). The new compound 1, quercitrin (3), and rutin (6) had antioxidant potential (EC50 values ranging from 8.1 to 13.6 µg/mL) comparable to ascorbic acid, used as positive control (EC50 7.4 µg/mL). The ten other compounds showed low or no antioxidant activity. Generally, substitution patterns on the B-ring especially affected antioxidant potencies of the flavonoids 28. The 3’,4’-dihydroxy pattern is particularly important to the antiradical activity of a flavonoid. These trends are consistent with less active flavonoids (2, 4, and 7-11) possessing 3’-OH pattern. The active compounds 1, 3, and 6, shared a common aglycone di-OH substituted in the B-ring (quercetin) whereas compound 7 is tri-OH substituted in the B-ring (myricetin). Comparison of the antioxidant activity of compounds 6 and 7 sharing the same disaccharide rha indicated that the 3’,4’-di-OH pattern is most favorable for the activity than 3’,4’,5’-tri-OH pattern (Table 1). Comparison of the antioxidant activity of the quercetin glycosides 1, 3, 5, and 6 showed that the saccharide chain linked at C-3 might have an influence on the antioxidant activity (Table 1). The antioxidant activity of these quercetin glycosides may justify the use of this plant in the treatment of diseases due to oxidative stress.
Experiments
General Experimental Procedures
DPPH (1,1-diphenyl-2-picrylhydrazyl radical) and ascorbic acid used for the bioassay were purchased from Sigma-Aldrich, Chemical Co. (Germany). NMR spectra were carried in CD3OD on Bruker Avance DRX III 500 instruments (1H at 500 MHz and 13C-Jmod at 125 MHz). HR-ESI-MS experiments were performed using a Micromass Q-TOF micro instrument (Manchester, UK). Optical rotations were determined in MeOH with a Perkin-Elmer 341 polarimeter. TLC was performed on pre-coated silica-gel 60 F254 Merck. CC was carried out on Kieselgel 60 (63-200 mesh), or LiChroprep RP-18 (40-63 µm) Merck. HPLC was performed on a Dionex apparatus equipped with an ASI-100 autosampler, an Ultimate 3000 pump, a diode array detector UVD 340S and Chromeleon software. C18 reversed phase column (Phenomenex 250x15 mm, Luna 5µ) was used for semi-preparative HPLC with binary gradient eluent (H2O (pH 2.4 with TFA); MeCN) and a flow rate of 4 mL/min; the chromatogram was monitored at 205, 225, 250, and 350 nm. Absorbance (A) values in the DPPH free radical scavenging assay were read on a Fluostar omega microplate reader (BMG labtech). UV spectra were recorded on Shimadzu UV-2450 spectrophotometer in MeOH.
Plant Material
The aerial parts of S.afzelii were collected from Cocody-Abidjan, Ivory Coast, in December 2010. The plant was identified by Pr. Laurent AKE-ASSI of FHB University and a voucher specimen (No Aké-Assi 21253) has been deposited in the herbarium of the National Center of Floristic of FHB University of Cocody (Ivory Coast).
Extraction and Isolation
The powdered dry aerial part of S. afzelii(1 Kg) were macerated with 14 L of 80% aqueous MeOH and further refluxed for 3 h. After filtration, this extract was concentrated under reduced pressure using rotatory evaporator to 2 L and was successively extracted with petroleum ether (PE), ethyl acetate, and n-butanol (each 3 x 1 L). After evaporation of the solvents, 10.8 g of PE soluble part, 7.5 g of EtOAc soluble part and 18.7 g of n-BuOH soluble part were obtained.
The n-BuOH soluble part was subjected to VLC over RP-18 (9 x 5 cm) eluted successively with 25, 50, 75, and 100% MeOH in H2O, to give four fractions (B1-B4, respectively). Fraction B2 (2.9 g) was applied to a silica gel CC (2.5 x 20 cm) eluted with a gradient of CHCl3:MeOH (9:1→7:3) to afford 67 fractions, each 40 mL. Frs 41-43 eluted with CHCl3:MeOH (7:3) contain 36 mg of compound 9. Frs 34-40 (120 mg), eluted with CHCl3:MeOH (75:25), were purified by semi-prep HPLC using a gradient of MeCN:H2O (from 13 to 30%; 22 min) to afford compounds 10 (rt 9.1 min, 9 mg), 11 (rt 10.2 min, 8 mg), 8 (rt 13.6 min, 10 mg), and 2 (rt 16.3 min, 24 mg). Frs 48-51 (90 mg), eluted with CHCl3:MeOH (7:3), were purified by semi-prep HPLC using a gradient of MeCN:H2O (from 10 to 25%; 20 min) to afford compounds 7 (rt 7.2 min, 7 mg), 1 (rt 8.9 min, 5 mg), and 6 (rt 9.3 min, 10 mg).
The EtOAc extract was subjected to VLC over RP-18 (9 x 5 cm) eluted successively with 25, 50, 75, and 100% MeOH in H2O, to give four fractions (A1-A4, respectively). Fraction A3 (1.8 g) was applied to a silica gel CC (2 x 16 cm) eluted with a gradient of CHCl3:MeOH (10:0→7:3) to afford 60 fractions, each 30 mL. Frs 20, 21, 22, 23, 24, 25 (83 mg), eluted with CHCl3:MeOH (8:2) were purified by semi-prep HPLC using a gradient of MeCN:H2O (from 18 to 30%; 20 min) to yield compounds 3 (rt 7.5 min, 11 mg), 5 (rt 10 min, 8 mg), and 4 (rt 13.5 min, 9 mg).
Acid Hydrolysis
A part of fractions A3 and B2 (200 mg each) was refluxed (90 °C) with 50 mL of 2M TFA for 4 h. After extraction with ethyl acetate (3 x 15 mL), the aqueous layer was evaporated to furnish the monosaccharides residue (200mg). Four sugars were identified as apiose, glucose, galactose and rhamnose by comparison with authentic samples on TLC in MeCOEt:iso-PrOH:acetone:H2O (20:10:7:6) 29. A part of the monosaccharides residue (50 mg) was subjected to a preparative TLC using the same solvent. The optical rotation of each purified sugar was measured to reveal l-rhamnose, d-glucose, d-galactose, and d-apiose 29.
Quercetin-3-O-β-d-Apiofuranosyl-(1→2)-α-l-Rhamnopyranoside(1)
Genkwanin-8-C-β-d-Apiofuranosyl-(1→2)-β-d-Glucopyranoside (11)
DPPH Free Radical Scavenging Assay
All tested compounds 1-11 showed on HPLC purity of more than 95%. The free radical scavenging activity of the extracts and isolated compounds against DPPH was investigated by spectrophotometric methodology, as previously described 8. Briefly, 5 µL of the standard or sample solutions (dissolved in DMSO) was mixed with 95 µL of DPPH solution (158 µM, dissolved in absolute EtOH). After mixing gently and incubating for 30 min at 37 °C, the optical density was measured at l 515 nm using a Fluostar omega microplate reader (BMG labtech). The percentage of absorbance inhibition at l 515 nm was calculated using the following equation: % inhibition (Acontrol - Asample/Acontrol) × 100. DPPH solution in EtOH was used as a control. The curve of the % scavenging activity against the concentration of sample was prepared by an MSExcel based program to obtain the EC50 (concentration required to obtain a 50% antioxidant effect). All the tests were conducted in triplicate. The experimental data were expressed as mean ± standard deviation.
Acknowledgements
The authors are grateful to CNRS, Conseil Régional Champagne Ardenne, Conseil Général de la Marne, and Ministry of Higher Education and Research (MESR), and to the PlANET CPER project for financial support.
References
- 1.Shahidi F, Ambigaipalan P. (2015) Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects –A review. , J. Funct. Foods 18, 820-897.
- 2.Lichtenberg D, Pinchuk I. (2015) Oxidative stress, the term and the concept. , Biochem. Biophys. Res. Commun 46, 441-444.
- 4.J M Mates, F M Sanchez-Jimenez. (2000) Role of reactive oxygen species in apoptosis: implications for cancer therapy. , Int. J. Biochem. Cell Biol 32, 157-170.
- 5.H N Siti, Kamisah Y, Kamsiah J. (2015) The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). , Vascul. Pharmacol 71, 40-56.
- 6.Bendaikha S, Gadaut M, Harakat D, Magid Alabdul, A. (2014) Acylated flavonol glycosides from the flower of Elaeagnus angustifolia L. , Phytochemistry 103, 129-136.
- 7.Sientzoff P, Hubert J, Janin C, Voutquenne-Nazabadioko L, J H Renault et al. (2015) Fast identification of radical scavengers from Securigera varia by combining 13C NMR based dereplication to bioactivity-guided fractionation. , Molecules 20, 14970-14984.
- 8.Gossan D P A, Magid Alabdul, Akoua Yao-Kouassi A, Ahibo P, Coffy A et al. (2015) New acylated flavonol glycosides from the aerial parts of Gouania longipetala. , Phytochem. Lett 11, 306-310.
- 10.T A Abere, D N Onwukaeme. (2012) Pharmacognostic evaluation of the leaves of Secamone afzelii. , (Schult) K Schum (Asclepiadaceae). Trop. J. Pharm. Res 11, 125-131.
- 11.P J Houghton, P J Hylands, A Y Mensah, Hensel A, A M Deters. (2005) In vitro tests and ethnopharmacological investiga-tions: wound healing as an example. , J. Ethnopharmacol 100, 100-107.
- 12.A Y Mensah, P J Houghton, G N Akyirem, T C Fleischer, M L et al. (2004) Evaluation of the antioxidant and free radical scavenging properties of Secamone afzelii Rhoem. Phytother Res. 18, 1031-1032.
- 13.A Y Mensah, Mireku E A Okwuonu, V. (2014) Anti-inflammatory and anti-oxidant activities of Secamone afzelii (Rhoem). , Asclepiadaceae. J. Med. Biomedical Sci 1, 23-30.
- 14.Zabri H, Kodjo C, Benie A, Bekro Mamyrbekova, Bekro J et al. (2008) Phytochemical screening and determination of flavonoids in Secamone afzelii (Asclepiadaceae) extracts. , Afr. J. Pure Appl. Chem 2, 80-82.
- 15.S C Lin, S F Tasi, S. (2011) Flavonoid glycosides from the leaves of Machilus philippinensis. , J. Chinese Chem. Soc 58, 555-562.
- 16.M, Mahmoud M A A, El-Nahas H A K, El-Toumy S A H, E A et al. (2010) Bio-guided isolation and structure elucidation of antioxidant compounds from the leaves of Ficus sycomorus. , Pharmacologyonline 3, 317-332.
- 17.Kazuma K, Noda N, Suzuki M. (2003) Malonylated flavonol glycosides from the petals of Clitoria ternatea. , Phytochemistry 62, 229-237.
- 18.Brasseur T, Angenot L. (1986) Flavonol glycosides from leaves of Strychnos variabilis. , Phytochemistry 25, 563-564.
- 19.Yasukwa K, M A Takido. (1987) flavonoid glycoside from Lysimachia mauritiana. , Phytochemistry 26, 1224-1226.
- 20.Lu Y, L Y Foo. (2000) Flavonoid and phenolic glycosides from Salvia officinalis. , Phytochemistry 55, 263-267.
- 21.S W Chang, K H, I K Lee, S U Choi, S Y Ryu et al. (2009) Phytochemical constituents of Bistorta manshuriensis. , Nat. Prod. Sci 15, 234-240.
- 22.Güvenalp Z, Demirezer O. (2005) Flavonol glycosides from Asperula arvensis L. , Turk J Chem 29, 163-169.
- 24.Ishii T, Yanagisawa M. (1998) Synthesis, separation and NMR spectral analysis of methyl apiofuranosides. , Carbohydr. Res 313, 189-192.
- 25.J H Zou, J S Yang, Zhou L. (2004) Acylated flavone C-glycosides from Trollius ledebouri. , J. Nat. Prod 67, 664-667.
- 26.C M Ma, Nakamura N, Hattori M. (1998) Saponins and C-glycosyl flavonoids from the seeds of Abrus precatorius. , Chem. Pharm. Bull 46, 982-987.
- 27.W S Feng, Y J Li, X K Zheng, Y Z Wang, F Y Su et al. (2011) Two new C-glycosyl flavones from Boea hygrometrica. , J. Asian Nat. Prod. Res 13, 618-623.
Cited by (3)
- 1.Oppong Bekoe Emelia, Kitcher Cindy, Ama Mireku Gyima Nana, Schwinger Gladys, Frempong Mark, 2019, , , (), 10.5772/intechopen.82199
- 2.Supasuteekul Chonlakan, Nuamnaichati Narawat, Mangmool Supachoke, Likhitwitayawuid Kittisak, Tengamnuay Parkpoom, et al, 2017, Antioxidant Activity and Upregulation of Antioxidant Enzymes of Phenolic Glycosides fromAquilaria crassnaLeaves, Natural Product Communications, 12(11), 1934578X1701201, 10.1177/1934578X1701201108
- 3.Benmerache Abbes, Alabdul Magid Abdulmagid, Kabouche Ahmed, Harakat Dominique, Voutquenne-Nazabadioko Laurence, et al, 2020, 6"'-O-acetylisospinosin, a new C-glycosylflavone and known compounds from the aerial parts of Cladanthus mixtus, Natural Product Research, 34(20), 2887, 10.1080/14786419.2019.1596100
