Abstract
Reactive oxygen species (ROS) and reactive nitrogen species are believed to be one of the most important culprits in the pathogenesis of cardio/cerebrovascular diseases. Intensive researches have been conducted to target free radicals as potential treatment for cardio/cerebrovascular diseases. The 2-(((1,1-dimethylethyl) oxidoimino)-methyl)-3,5,6-trimethylpyrazine (TBN), a novel nitrone derivative of tetramethylpyrazine, has been demonstrated to exhibit significant therapeutic effects in ischemic stroke and Parkinson’s models due to its multiple functions, including calcium overload blockade and free radical-scavenging activity. In the present study, we found that TBN had significant radical trapping effect in cell-free assays. Additionally, TBN effectively blocked tert-butylhydroperoxide (t-BHP)-induced murine H9c2 cardiomyoblast cell death, suppressed H9c2 cell apoptosis and reversed the decrease in mitochondrial membrane potential. Furthermore, TBN markedly inhibited t-BHP-induced ROS generation and free radical NO and ONOO–.Taken together, these results suggest that TBN might be a potential candidate for the treatment of ischemic cardio/cerebrovascular diseases by targeting free radicals.
Author Contributions
Academic Editor: Suowen Xu, University of Rochester
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Longjun Zhu, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are a family of molecules that include molecular oxygen and its derivatives produced in all aerobic cells. They usually act as second messengers in cell signaling that are essential for various biological processes in normal cells1. It has been well documented that they regulate many signal transduction pathways by directly reacting with and/or modifying the structures of proteins, enzymes, transcription factors and genes to modulate their functions.
Reactive oxygen species ROS, which are mainly generated in mitochondria, includes three types: superoxide anion radical (O2–), hydrogen peroxide (H2O2) and hydroxyl radical (OH), Approximately 1-3% of the oxygen taken up by the cell escapes from the mitochondrial electron transport chain, and is constitutively present in the form of O2– 2, 3, 4 O2 is subsequently converted to H2O2 by dismutation of O2– or directly from the action of oxidase enzymes. Hydroxyl radical (OH), a highly reactive species that can be converted from H2O2 by Fenton reaction, will modify base pairs and cause strand breaks and result in DNA damage. RNS, often refers to nitric oxide (NO) and peroxynitrite (ONOO–). Nitric oxide radical (NO) can arise from L-arginine catalyzed by cytosolic or mitochondrial nitric oxide synthases (NOS) 5, while in the presence of O2–, NO will react with O2– instantly to form peroxynitrite (ONOO–).
It has been demonstrated that ischemia is a restriction of blood supply generally due to congestion, as the limitation of blood flow to the tissue eventually culminates cell damage. However, the re-introduction of oxygen to the ischemic tissues results in a burst of ROS and RNS production, which can subsequently cause fatal damage in cellular components. This type of tissue damage is referred to as ischemia reperfusion injury6. Any aberrance in reactive species, in particular those derived from NO and O2–, have been shown to cause cellular oxidative damage and trigger specific signaling events that culminate in altered cellular physiology7,8. Normal tissues have a defense system against these toxic ROS and RNS, however, ischemia reperfusion injury overwhelms the protective mechanisms and results in ROS and RNS burst, which is the culprit of the pathogenesis and/or progression of ischemic cardio/cerebro vascular disease.
Edaravone (3-methyl-1-phenyl-2-pyrazoline-5-one, Figure 1), designed and marketed as a neuroprotectant by Mitsubishi Tanabe Pharma Corporation (Tokyo, Japan)9, acts as a potent antioxidant and effective free radical scavenger against oxidative stress and neuronal apoptosis10, 11, 12, and is used for the purpose of aiding neurological recovery following acute ischemic cerebral infarction10,13. Besides, it also exhibits preventive effects on myocardial injury in patients with acute myocardial infarction14,15.
Tetramethylpyrazine (2,3,5,6-tetramethylpyrazine, TMP, Figure 1) is the main active ingredient of traditional Chinese medicine Ligusticum wallichii Franchat (Chuan Xiong). It has been demonstrated that TMP exerts potential radical-scavenging and anti-oxidative activities in vitro 16, 17, 18, and has been used in the therapy of cerebral ischemic disease 19, 20 and myocardial ischemia-reperfusion injury 21, 22.
Figure 1.The chemical structures of Edaravone, TMP, NXY-059, and TBN.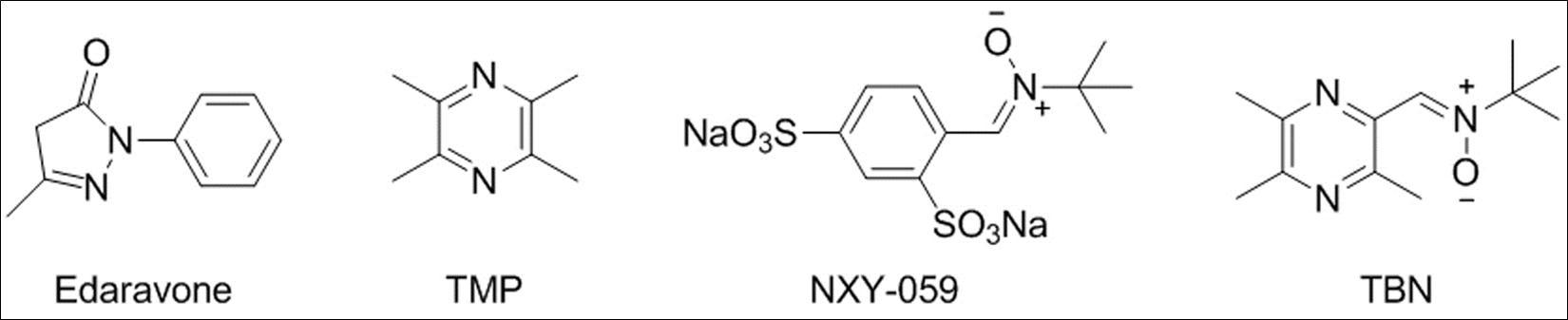
Disodium (tert-butylimino) methyl) benzene-1, 3-disulfonate N-oxide (NXY-059, Figure 1), a disulfonyl derivative of spin trap α-phenyl-tert-butyl nitrone (PBN) 23, 24 that has potent radical-trapping property, was designed as a neuroprotective compound for ischemic stroke 25, 26, 27. Although the SAINT III (Stroke-Acute Ischemic NXY Treatment III) clinical trial failed in 2006 28, the concept of using radical-trapping property of nitrone moiety as neuroprotective agents for ischemic reperfusion injury therapy remains viable.
TBN (2-[(11-methyl]-3, 5, 6-trimethylpyrazine, Figure 1), a TMP derivative armed with a powerful nitrone moiety, was designed as a dual-functional agent targeting overload of calcium and free radicals by our group 29. We had previously demonstrated that TBN possessed significant free radical-scavenging activity against various radicals, including hydroxyl (OH), superoxide (O2−) and peroxynitrite (ONOO−) 29, 30. Furthermore, we have also revealed that TBN remarkably protects neuronal cells from oxidative injury in vitro 29 and rats from ischemic stroke 29, 31.
Due to the high reactivity of reactive species with the surrounding biological macromolecules and relatively short duration of existence, the determination of ROS and RNS scavenging effects of target compounds in intracellular compartments is difficult 8. Up to now, there were very few systematic evidence demonstrated the radical scavenging effectiveness among edaravone, TMP and NXY-059. Herein, we determine the radical-scavenging and antioxidant effects of TBN in comparison with edaravone, TMP and NXY-059 in vitro, providing experimental evidence for identifying TBN as a potential therapeutic agent of ischemic cardio/cerebrovascular disease.
Materials and Methods
Chemicals and Reagents
TBN and NXY-059 were synthesized and purified in our laboratory as described previously 32, 33, 34. TMP, edaravone 2,2-diphenyl-1-picrylhydrazyl (DPPH), N,N-dimethyl-4-nitrosoaniline (p-NDA), H2O2, pyrogallol, 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris), 3-aminophthalhydrazide (luminol), tert-butyl hydroperoxide (t-BHP), 3-morpholinosydnonimine hydrochloride (SIN-1) and diethylamine NONOate diethylammonium salt (DEA NONOate) were purchased from Sigma Aldrich (St Louis, Mo, USA). Hoechst 33342, MitoProbe JC-1 assay kit, MitoSOX™ Red Mitochondrial Superoxide Indicator, 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA), dihydrorhodamine 123 (DHR 123), 4-amino-5-methylamino-2',7'-difluorofluorescein diacetate (DAF-FM DA), Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 0.25% Trypsin-EDTA, Penicillin-Streptomycin (10,000 U/ml), phosphate buffered saline (PBS), were obtained from Invitrogen, Life technologies (Carlsbad, CA, USA). All other reagents were purchased from Sigma Aldrich unless otherwise specified.
Determination of Radical-Trapping Activity Against DPPH, OH, O2− and ONOO− by Cell-Free Assays
The comparison of free radical-trapping activities among TBN, TMP, NXY-059 and edaravone (5, 20, 80, 320 μM used for all compounds) against 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), hydroxyl radical (OH), superoxide anion (O2−) and peroxynitrite (ONOO−) were determined by cell-free assays according to the previously published procedures with minor modification 31, 35.
DPPH radical-scavenging activity. One hundred microliter methanol (control) or a methanolic solution of each test compound was added in 96-well plates, and then 100 μl methanolic solution of DPPH (final concentration 50 μM) was added into each well. The plates were incubated in the dark at room temperature for 50 min. The measurement at 517 nm was measured using a plate reader (BioTek Synergy 4, Winooski, Vermont, USA). The clearance of the DPPH radical was calculated as follows: Clearance (%)= [(Actrl–At) /Actrl] × 100. Where Actrl was the absorbance of the control, and At was the absorbance of each sample solution at the time t = 50 min.
Hydroxyl radical-scavenging activity. The p-NDA, FeSO4 and H2O2 were freshly prepared in N2-purged, double-distilled H2O (ddH2O) to get a concentration of 1.0 mM, 2.0 mM and 1.0 mM respectively. While all of the test compounds were freshly dissolved in ddH2O before the experiment, then 300 μl ddH2O (control) or different concentration of each test compound, 50 μl p-NDA, 125 μl H2O2 and 125 μl Fe2+ were added to48-well plate in order. Hydroxyl free radical was generated by the Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH‒ + OH). The bleaching of p-NDA was monitored as the loss in absorbance at 440 nm for 100 s on a BioTek Synergy 4. The Clearance was calculated as follows: Clearance (%)=[1 ‒ (A0 ‒ A100)/A0] ×100, where A0 is the absorbance at the time t=0 s, and A100 is the absorbance at t=100 s.
Superoxide anion-scavenging activity. Pyrogallol was dissolved in 10 mM HCl solution while Tris-HCl was prepared in N2-purged ddH2O to get a concentration of 2.0 mM and 50 mM, respectively. In each well of a 48-well plate, 250 μl Tris-HCl buffer (pH 8.2), 300 μl ddH2O or different concentration of each test compound, 50 μl pyrogallol (final concentration 0.17 mM) was added one after another. The autoxidation rate of pyragallol was presented as the increase of absorbance per second at 320 nm (dA/dt) for 300 s on a BioTek Synergy 4. The inhibition ratio of pyrogallol autoxidation was calculated according to the formula: Clearance (%) = (dActrl/dt ‒ dAsample/dt) /dActrl/ dt ×100.
Peroxynitrite-scavenging activity. Luminol and SIN-1 were dissolved in ice-water bathed PBS separately to get a concentration of 1.0 mM and 3 mg/ml. Firstly, 150 μl PBS, 250 μl of PBS (control) or each test compound, 50 μl luminol were pipetted into the luminometer tube before been positioned into the measurement chamber. Finally the reaction was initiated by adding 50 μl ice-colded SIN-1 with an injector. Then the luminescence was measured on an ultra-sensitive tube luminometer (Berthold Lumat LB 9507, Bad Wildbad, Germany) at the time interval of 100 s for 2000 s. The clearance at peak height represented the compounds’ ability of trapping ONOO–. The clearance was calculated as follows: Clearance (%) = [(Actrl ‒ Asample) /Actrl] × 100.
Cell Culture
Murine H9c2 cardiomyoblasts (ATCC CRL-1446, Rockville, MD, USA) were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (100 U/ml), at 37 oC in a humidified atmosphere with 5% CO2. Cells were passaged regularly and sub-cultured to ∼80% confluence, then starved with serum free DMEM for 24 h before experimental procedures.
Effects of t-BHP, DEA NONOate and SIN-1 on Cells Viability of H9c2
H9c2 cells were seeded in the 96-well plate (5×103 cells/well) for 24 h growth, then exposed to increasing doses of t-BHP, DEA NONOate or SIN-1 for 24 h. The impairment of cell viability was determined by MTT method as described previously36.
Effects on t-BHP-Induced Cell Death in H9c2 Cells
In order to determine the effects of target compounds on t-BHP-induced cell death, the H9c2 cells were seeded in the 96-well plate (5×103 cells/well) for 24 h growth, then pretreated with NXY-059, TMP, TBN (10, 300, 100, 300 μM) or edaravone (5 μM) for 2 h, respectively, before t-BHP was added for another 24 h incubation to induced cell damage. Cell viability was measured by MTT method as described previously36.
Evaluation of Nuclei Morphological Changes of H9c2 cells by Hoechst 33342 Staining
The H9c2 cells were cultured on cover slips (1×104 cells) in 6-well plates and starved for 24 h, pretreated with 300 μM TBN or edaravone for 2 h, and then 150 μM t-BHP was added to the culture for another 24 h. Following fixation with 1% formalin for 10 min at room temperature, Hoechst 33342 (10 µg/ml) was added to the cover slips for 30 min under dark condition to stain the nuclei. Cells were observed and photographed under a fluorescence microscope (Olympus IX71, Shibuya-ku, Tokyo, Japan). Tetraplicate cells were prepared in each experimental condition. Apoptotic indices were determined by direct visualization and counting of a minimum of at least 200 cells from five randomly selected fields in each treatment, and expressed as a percentage of the total number of nuclei counted. The apoptotic index was calculated as the ratio of number of apoptotic cells to total cells counted in the field ×40 objective37.
Assessment of Mitochondrial Membrane Potential in H9c2 Cells
The mitochondrial membrane potential (Δψm) was determined by a MitoProbe™ JC-1 assay kit (Invitrogen). Briefly, H9c2 cells seeded in Costar 96-well clear bottom black side microplate (5×103 cells/well) were pretreated with or without 0.1, 1, 10, 100 μM target compounds for 2 h and then exposed with 150 μM t-BHP for another 1 h. Subsequently, the medium was replaced with 2 μM JC-1 and incubated for 30 min at 37 oC, 5% CO2. The unbound dye was removed by washing with PBS twice, while the fluorescence of J-monomer and J-aggregates were quantified under area scan mode on a BioTek Synergy 4. The result was presented as the ratio of red/green fluorescence values relative to the untreated control. Carbonyl cyanide m-chlorophenylhydrazone (CCCP, 10 μM) served as a positive control for this experiment.
Determination of Hydrogen Peroxide Scavenging Effect Induced by t-BHP in H9c2 Cells
H9c2 cells seeded in Costar 96-well clear bottom black side microplate (5×103 cells/well) were pretreated with or without 0.1, 1, 10, 100 μM target compound for 2 h, then 150 μM t-BHP was added to the culture for another 1 h. hydrogen peroxide scavenging activity was detected by employing 10 μM H2DCF-DA incubated in the dark at 37 oC for 30 min. The relative fluorescence intensity was analyzed under area scan mode on BioTek Synergy 4.
Determination of Anti-Superoxide Anion Activity Induced by t-BHP in H9c2 Cells
MitoSOX Red mitochondrial superoxide indicator, a highly selective fluorogenic dye for detection of superoxide in the mitochondria of live cells, exhibits red fluorescence upon oxidation by superoxide 38. H9c2 cells seeded in Costar 96-well clear bottom black side microplate (5×103 cells/well) were pretreated with or without 0.1, 1, 10, 100 μM target compound for 2 h, followed by exposing to 150 μM t-BHP for another 1 h. Subsequently, the culture medium was replaced by 5 μM MitoSOX Red in the dark at 37 oC for 30 min. Detection of oxidative MitoSOX Red was performed on a BioTek Synergy 4. The fluorescence intensity of control served at 100%, while the increased ratio of the relative fluorescence intensity presented as the amount of superoxide in the cells.
Determination of NO and ONOO– Scavenging Activity in H9c2 Cells
DEA NONOate can release NO in a controlled manner under physiological conditions nothing to do with the cell type and cellular enzyme 39, 40, while SIN-1 generates NO and O2– in equimolar amounts, and then forms the very reactive ONOO–41, 42. DAF-FM DA and Dihydrorhodamine 123 (DHR 123) were used as selective fluorogenic dye for detection of NO 43, 44 and ONOO–45, 46 in this experiment, respectively.
DEA NONOate and SIN-1 were dissolved in water at a final concentration of 50 mM each and store in small aliquots under - 80 oC until use. H9c2 cells seeded in Costar 96-well clear bottom black side microplate (5×103 cells/well) were pretreated with or without 25, 50, 100, 200 μM target compounds for 2 h, then co-incubated with 1500 μM DEA NONOate or 1600 μM SIN-1 in serum-free DMEM for another 1 h. Following this, the medium was replaced with 5 μM DAF-FM DA or 10 μM DHR 123 in the dark at 37 oC for 30 min. After washing twice with PBS, the fluorescent intensity were quantified under area scan mode on a BioTek Synergy 4. The increased ratio of relative fluorescence intensity compared to control group presented as the encasement amount of RNS in the cells.
Statistical Analysis
Each assay was carried out three times in order to determine the reproducibility. Data were expressed as mean ± standard deviation (SD) and statistical calculations were performed using prism 6.0 GraphPad software (GraphPad, San Diego, CA, USA). Comparisons between the different groups were performed by one-way analysis of variance (ANOVA) followed by Turkey’s test. p < 0.05 were considered significant.
Results
Free Radical-Scavenging Effect in Cell-Free Assays
The results showed that the effect on various radical-trapping among four different compounds was concentration-dependent from 5 to 320 μM. The radical-scavenging effects of TBN were more potent than TMP and NXY-059 against DPPH Figure 2A, O2– Figure 2B and ONOO– Figure 2C at the same concentration, even close to edaravone. However, TBN was slightly better than TMP, while weaker than NXY-059 and edaravone in terms of OH-trapping activity Figure 2D.
Figure 2.Free radical trapping and antioxidant effects of TBN in cell free systems.
Effects of t-BHP, DEA NONOate or SIN-1 on H9c2 Cell Viability and Comparison of Cytoprotection of TMP and NXY-059, TBN Against t-BHP Induced Cell Death
As shown in Figure 3A, all the three inducers impaired H9c2 cells viability in a concentration dependent manner. A reduction of 50.5 ± 3.6% was observed with 150 μM t-BHP, 46.4 ± 5.5% with 1600 μM DEA NONOate, and 48.0 ± 3.5% with 1600 μM SIN-1. We chose the concentration of t-BHP 150 μM, DEA NONOate 1500 μM and SIN-1 1600 μM in our subsequent experiments. TBN significantly ameliorated the impairment of 150 μM t-BHP on H9c2 cells Figure 3B. Compared to TMP and NXY-059, TBN was more efficient than TMP and NXY-059 against t-BHP induced cell death. Edaravone (5 μM) served as a positive control, also mildly increased the cell viability.
Figure 3.Effect of t-BHP, SIN-1 and DEA NONOate on H9c2 cell viability (A). H9c2 cell viability was decreased by t-BHP (10-800 μM), SIN-1 (10-2000 μM) and DEA NONOate (10-2000 μM) in a concentration-dependent manner which measured by MTT method. Comparison of cytoprotection against t-BHP induced cell death (B). H9c2 cells were pretreated with NXY-059, TMP, TBN (10-300 μM) or edaravone (5 μM) for 2 h, respectively. Then cells were co-incubated with 150 μM t-BHP for another 24 h. The cell viability was measured by MTT method. ###p < 0.001 vs control group. *p < 0.05, **p < 0.01 compared to 150 μM t-BHP group. The results were the mean ± SD of three independent experiments.
Evaluation of Nuclei Morphological Change of H9c2 Cells by Hoechst 33342 Staining
Apoptotic cells were identified as chromatin condensation or nuclear fragmentation with nucleus exhibiting brightly stain of Hoechst 33342. Most nuclei in the control displayed uniform blue chromatin with organized structure, while cells stimulated with t-BHP were identified as those with a nuclei exhibiting brightly stain of condensed chromatin or nuclear fragments Figure 4A. NXY-059, TMP, TBN or edaravone pretreatment all markedly ameliorated t-BHP-induced apoptosis Figure 4B.
Figure 4.Evaluation of cell apoptosis against t-BHP. (A) Cell morphological changes were evaluated by Hoechst 33342 staining. Apoptotic cells were observed and photographed by the inverted fluorescent microscopy (under 40× objective), identified as those with a nucleus exhibiting brightly stained condensed chromatin (long arrowheads) or nuclear fragments (short arrowheads). (B) The apoptosis index was determined by calculating the percentage of Hoechst-positive cells over the total number of cells. Significant: *p< 0.05, **p< 0.01, vs t-BHP only group. ## p< 0.01 vs control group. The results were the mean ± SD of four independent experiments.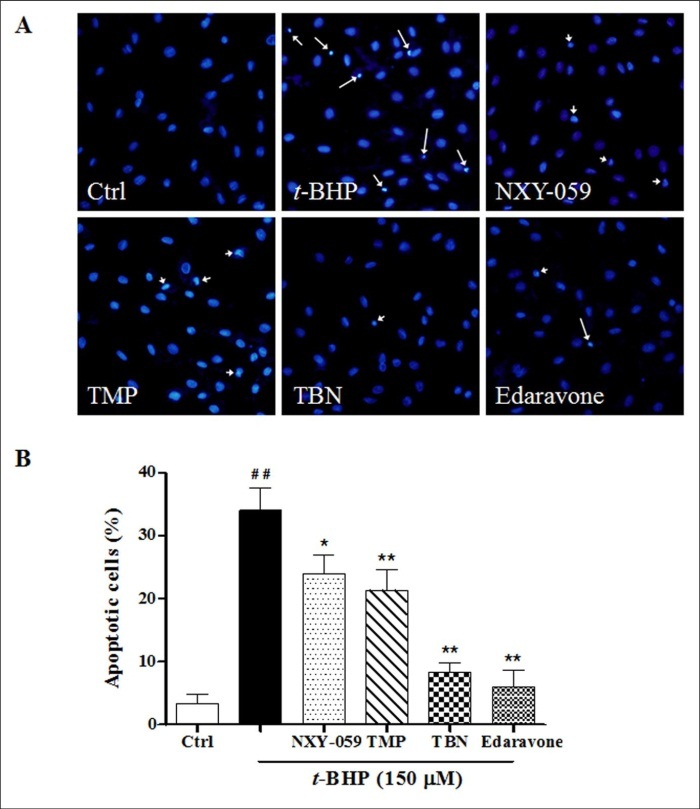
Assessment of the Mitochondrial Membrane Potential in H9c2 Cells
CCCP (10 μM) caused quick mitochondrial membrane depolarization, created a strong, single positive green fluorescence, served as a control. The pretreatment of all the four compounds could efficiently and concentration dependently reverse the Δψm loss caused by t-BHP. Compared to TMP and NXY-059, TBN was more potent in reversing the Δψm loss impaired by t-BHP Figure 5.
Figure 5.Evaluation of mitochondrial membrane potential against t-BHP impairment. CCCP served as a positive control. Data were presented as ratio of red / green fluorescence intensity relative to t-BHP treated only group. Significant: *p< 0.05, **p<0.01 vs t-BHP only group. ### p < 0.001 vs control group. The results were the mean ± SD of four independent experiments.
Intracellular H2O2 and Mitochondrial O2– Trapping Effect in H9c2 Cells
All four compounds significantly decreased the elevation of intracellular H2O2 and mitochondrial O2– stimulated by t-BHP in H9c2 cells. TBN was superior to NXY-059 and TMP against intracellular H2O2 and mitochondrial O2– induced by t-BHP at the same concentration, it was even better than the effect of edaravone at higher concentration Figure 6A and B.
Figure 6.Radical scavenging activity against (A) hydrogen peroxide and (B) superoxide anion induced by t-BHP. Data were presented as the increased ratio of relative fluorescence intensity compared to control, the ratio of all columns minus control group while treating control as 100%. Significant: ### p<0.001 vs control. *p<0.05, ** p<0.01, *** <0.001 vs t-BHP only group. The results were the mean ± SD of four independent experiments.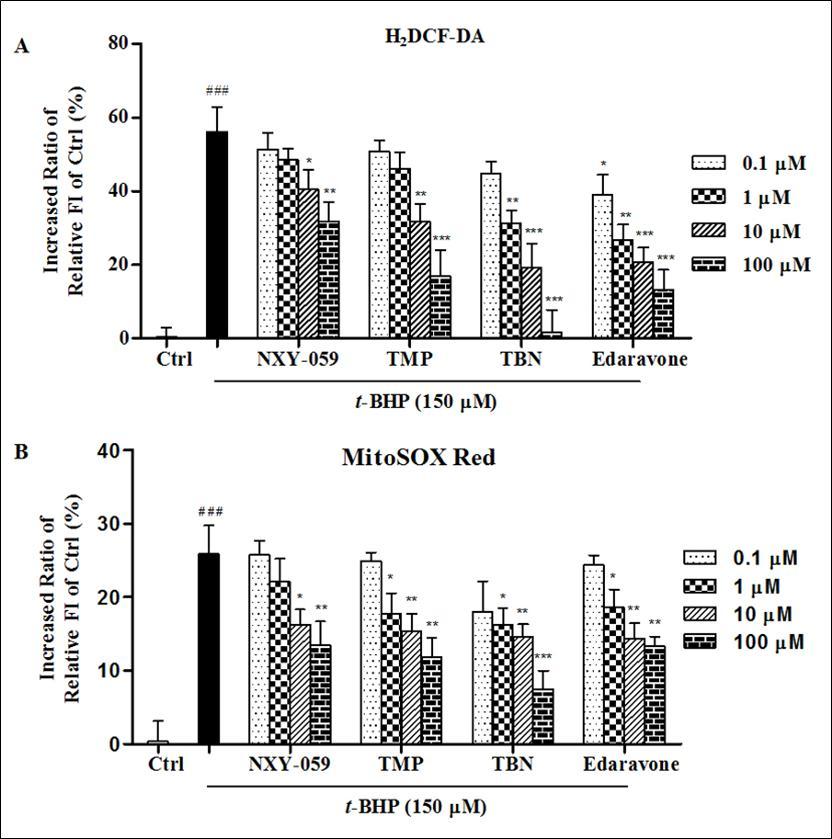
Radical-Trapping Activities Against NO and ONOO– in H9c2 Cells
In the NO-scavenging assay, the fluorescent intensity of the DEA NONOate treated alone group increased 29.3 ± 5.3% compared to the untreated control. Before exposure to DEA NONOate, 2 h pretreatment with all the four target compounds intensively decreased the NO level in H9c2 cells. TBN was weaker to edaravone but stronger to TMP and NXY-059 in NO-trapping activity Figure 7A. In the ONOO– trapping activity, the fluorescent intensity of the SIN-1 treated alone group increased 287.6 ± 12.1% compared to the untreated control. Pretreatment with target compounds significantly and concentration-dependently decreased the relative fluorescence intensity of DHR 123. Similar to the NO-scavenging activity, TBN was also weaker to edaravone but stronger than TMP and NXY-059 in ONOO–-trapping activity Figure 7B.
Figure 7.Radical-trapping effects against (A) NO and (B) ONOO– in H9c2 cells. Data were presented as the increased ratio of relative fluorescence intensity compared to control, the ratio of all columns minus control group while treating control as 100%. Significant: ### p < 0.001 vs control. * p < 0.05, ** p < 0.01, *** < 0.001 vs Model. The results were the mean ± SD of at least three independent experiments.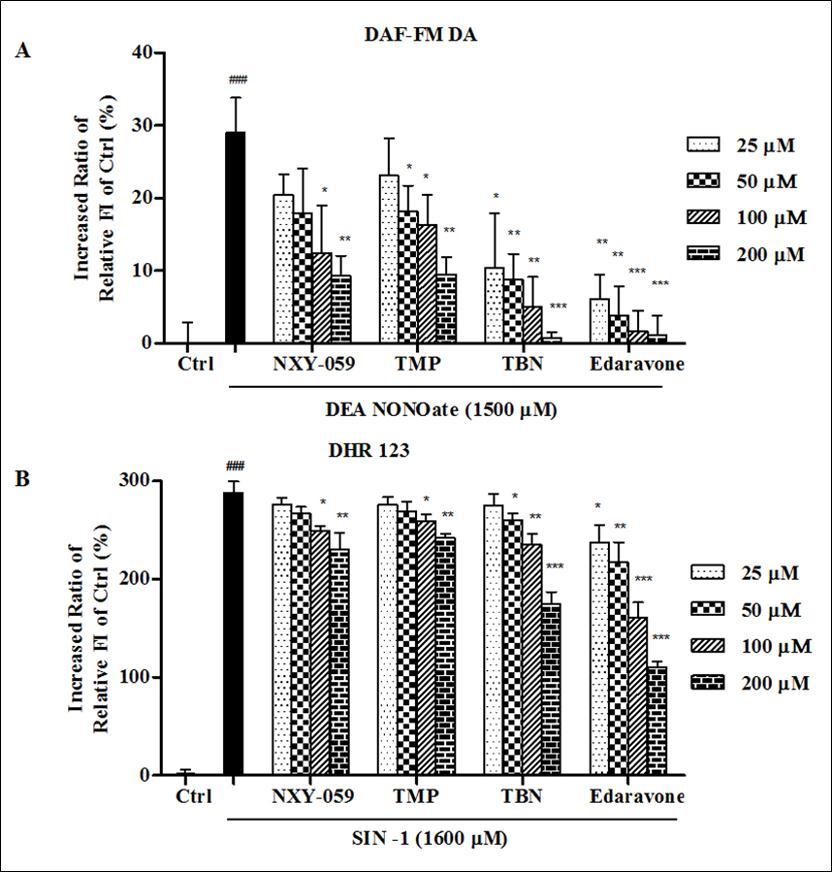
Discussion
In the present study, we first compared the free radical trapping activity of TBN with NYX-059, TMP and edaravone in cell-free assays , and demonstrated that TBN was weaker to edaravone but stronger to NXY-059 and TMP in DPPH, OH, O2– and ONOO– trapping.
In terms of the free radical trapping mechanism in cell-free assays, the reason of TMP against OH in Fenton reaction may be related with the formation of chelate with Fe2+, or the two nitrogen atoms of the pyrazine ring can provide lone pair of electrons to combine with OH. Besides, TMP may perform O2–-scavenging effect by catalyzing spontaneous dismutation of O2– to produce H2O2 and O2. Therefore, it could be speculated that the structure of TMP has two nitrogen with a pair of free electron, which could be oxidized by active oxygen free radicals that contribute to the reactivity of TMP 16. The reaction of the free radical species with a nitrone yields a free radical intermediate termed the spin adduct. NXY-059, a derivative of nitrone, performs its radical-trapping effect through forming nitrone-radical adducts47. TBN possessed superior free radical-scavenging activity to TMP and NXY-059 in these cell-free assays, and the precise reasons remains to be further explored.
Edaravone was the most potent and efficacious radical-scavenging compound among the four against DPPH, OH, O2– and ONOO– in these cell-free assays. Though edaravone has three tautomeric forms, the amine, keto, and enol forms, approximately 50% of edaravone exist in the anionic form at physiological pH because of the pKa value of edaravone is 7.0. A hypothetical radical-scavenging mechanism of edaravone has been reported previously 48.
Furthermore, we systematically compared the radical-scavenging and anti-oxidative activities of these four target compounds in murine H9c2 cardiomyoblast cells. Similar to the cell-free assays. TBN was weaker to edaravone in some aspects but more potent and efficaciously than NXY-059 and TMP in H2O2, O2–, NO and ONOO– trapping. However, the free-radical-scavenging effect of a compound in vivo or ex vivo is not only related to its chemical structure, but also associated with its molecular weight and octanol-water partition coefficient 49, 50. NXY-059 is a highly water-soluble molecule (MW: 381.33, ClogP: 0.95), and thus could not readily penetrate cell membrane to exert its radical-trapping activity in cells. NXY-059 showed positive results when evaluated in various animal stroke models but failed its second phase III clinical trial 26, 51, 52. The primary reason why NXY-059 failed could be due to its difficulty in penetrating the blood-brain barrier (BBB). Negatively charged compounds cannot readily cross the BBB, and NXY-059 has two sodium sulfonate moieties. TMP (MW: 136.19, ClogP 1.58), edaravone (MW: 174.20, ClogP 1.66) and TBN (MW: 221.30, ClogP: 1.55) have the appropriate molecular weight and octanol-water partition coefficient. They can smoothly penetrate cell membrane and BBB, and then perform the anti-radical effects.
Though the free radical-scavenging effect of TBN was shown a little weaker to edaravone in some aspects, TBN has a distinct advantage. In the clinical application, there are several lines of evidence showing that when the concentration of edaravone in the blood was >5 μM, severe hepatotoxicity was produced 53, 54. On the contrary, TBN demonstrates non-detectable toxicity even when the dose in blood exceeds 300 μM both in rat and marmoset primate model (data not shown). Besides, the concentration of TBN in brain tissues can reach approximately 160 μM when administered at 80 mg/kg in rats 31.
As an intracellular gaseous signaling molecules and cellular messenger, nitric oxide (NO) is synthesized from the amino acid L-arginine and O2, which plays key functional role in cardiovascular system, including maintenance of blood pressure and other cardiovascular function. However, excess of NO will drive progression of cardiovascular complication, especially when NO reacts with O2– instantly to form peroxynitrite (ONOO–)5. As suppression of excessive production of intracellular NO and ONOO–, TBN will benefit the cardio/cerebrovascular system.
In conclusion, the radical-trapping and neuroprotective effects of TBN described herein and previously 29, 31, 35 suggest that TBN might be a promising candidate for the treatment of ischemic cardio/cerebrovascular diseases.
Acknowledgement
This work is partially supported by grants from China’s ‘12.5’ Innovative Drug Project (2012ZX09103101-055 to YW), the Science and Technology Project of Guangdong (2012A080201009 to YW, 2013A022100030 to PY and 2015A030313317 to ZZ), as well as the Science and Technology Program of Guangzhou (2014J4100097 to ZZ)
References
- 1.Darley-Usmar V, Wiseman H, Halliwell B. (1995) Nitric oxide and oxygen radicals: a question of balance. , FEBS letters 369, 131-135.
- 2.Batandier C, Fontaine E, Keriel C, X M Leverve. (2002) Determination of mitochondrial reactive oxygen species: methodological aspects. , Journal of cellular and molecular medicine 6, 175-187.
- 3.A P Kudin, N Y Bimpong-Buta, Vielhaber S, C E Elger, W S Kunz. (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. , The Journal of biological chemistry 279, 4127-4135.
- 4.Liu Y, Fiskum G, Schubert D. (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. , Journal of neurochemistry 80, 780-787.
- 5.Poderoso J J. (2009) The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide, Archives of biochemistry and biophysics 484. 214-220.
- 6.Brookes P S, Yoon Y, Robotham J L, Anders M W, Sheu S S. (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle, American journal of physiology. , Cell physiology 287, 817-833.
- 7.R M Uppu, B D Nossaman, A J Greco, Fokin A, S N Murthy et al. (2007) Cardiovascular effects of peroxynitrite, Clinical and experimental pharmacology and physiology. 34, 933-937.
- 8.Bauer G. (2002) Signaling and proapoptotic functions of transformed cell-derived reactive oxygen species, Prostaglandins, leukotrienes, and essential fatty acids 66. 41-56.
- 9.Tabrizchi R. (2000) Edaravone Mitsubishi-Tokyo, Current opinion in investigational drugs. 1, 347-354.
- 10.Tanaka M. (2002) Pharmacological and clinical profile of the free radical scavenger edaravone as a neuroprotective agent. , Folia Pharmacologica Japonica 119, 301-308.
- 11.Watanabe T, Tanaka M, Watanabe K, Takamatsu Y, Tobe A. (2004) [Research and development of the free radical scavenger edaravone as a neuroprotectant], Yakugaku zasshi :. , Journal of the Pharmaceutical Society of Japan 124, 99-111.
- 12.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N et al. (2006) Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. , CNS drug reviews 12, 9-20.
- 13.Edaravone Acute Infarction Study,G. (2003)Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. , Cerebrovascular diseases 15, 222-229.
- 14.Tsujita K, Shimomura H, Kawano H, Hokamaki J, Fukuda M et al. (2004) Effects of edaravone on reperfusion injury in patients with acute myocardial infarction, The American journal of cardiology 94. 481-484.
- 15.Higashi Y, Jitsuiki D, Chayama K, Yoshizumi M. (2006) Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases, Recent patents on cardiovascular drug discovery. 1, 85-93.
- 16.Zhang Z H, S Z Yu, Wang Z T, Zhao B L, Hou J W et al. (1994) Scavenging effects of tetramethylpyrazine on active oxygen free radicals, Zhongguo yao li xue bao =. , Acta pharmacologica Sinica 15, 229-231.
- 17.Zhang Z, Wei T, Hou J, Li G, Yu S et al. (2003) Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. , Life sciences 72, 2465-2472.
- 18.W M Li, H T Liu, X Y Li, J Y Wu, Xu G et al. (2010) The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells, Basic and clinical pharmacology and toxicology 106. 45-52.
- 19.Jia J, Zhang X, Y S Hu, Wu Y, Q Z Wang et al. (2009) Protective effect of tetraethyl pyrazine against focal cerebral ischemia/reperfusion injury in rats: therapeutic time window and its mechanism. , Thromb Res 123, 727-730.
- 20.S L Liao, T K Kao, W Y Chen, Y S Lin, S Y Chen et al. (2004) Tetramethylpyrazine reduces ischemic brain injury in rats. , Neuroscience letters 372, 40-45.
- 21.Lv L, S, Xu J, J B Gong, Cheng Y. (2012) Protective effect of ligustrazine against myocardial ischaemia reperfusion in rats: the role of endothelial nitric oxide synthase, Clinical and experimental pharmacology and physiology 39. 20-27.
- 22.S Y Chen, Hsiao G, H R, P Y Cheng, Y M Lee. (2006) Tetramethylpyrazine induces heme oxygenase-1 expression and attenuates myocardial ischemia/reperfusion injury in rats. , Journal of biomedical science 13, 731-740.
- 23.Ohto N, Niki E, Kamiya Y. (1977) Study of autoxidation by spin trapping. Spin trapping of peroxyl radicals by phenyl N-t-butyl nitrone. , Journal of the Chemical Society, Perkin Transactions 2, 1770.
- 24.J A Howard, J C Tait. (1978) Electron paramagnetic resonance spectra of thetert-butylperoxy andtert-butoxy adducts to phenyltert-butyl nitrone and 2-methyl-2-nitrosopropane. Oxygen-17 hyperfine coupling constants. , Canadian Journal of Chemistry 56, 176-178.
- 25.K R Lees, J A Zivin, Ashwood T, Davalos A, S M Davis et al. (2006) NXY-059 for ac 2007) NXY-059 for the treatment of acute ischemic stroke, The New England journal of medicine 357. 562-571.
- 26.Kuroda S, Tsuchidate R, M L Smith, K R Maples, B K Siesjo. (1999) Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. , J Cereb Blood Flow Metab 19, 778-787.
- 27.Savitz S I, Fisher M. (2007) Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. , Annals of neurology 61, 396-402.
- 28.Sun Y, Jiang J, Zhang Z, Yu P, Wang L et al. (2008) Antioxidative and thrombolytic TMP nitrone for treatment of ischemic stroke. , Bioorg Med Chem 16, 8868-8874.
- 29.Sun Y, Yu P, Zhang G, Wang L, Zhong H et al. (2012) Therapeutic effects of tetramethylpyrazine nitrone in rat ischemic stroke models. , J Neurosci Res 90, 1662-1669.
- 30.Sun Y, Yu P, Zhang G, Wang L, Zhong H et al. (2012) Therapeutic effects of tetramethylpyrazine nitrone in rat ischemic stroke models. , Journal of neuroscience research 90, 1662-1669.
- 31.P A Lapchak, D M Araujo, Song D, Wei J, Purdy R et al. (2002) Effects of the spin trap agent disodium- [tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit Large clot embolic stroke model: combination studies with tissue plasminogen activator, Stroke; a journal of cerebral circulation. 33, 1665-1670.
- 32.R D Hinton, E G Janzen. (1992) Synthesis and characterization of phenyl-substituted C-phenyl-N-tert-butylnitrones and some of their radical adducts. , The Journal of Organic Chemistry 57, 2646-2651.
- 33.J M Carney. (1998) 2, 4-Disulfo phenyl butyl nitrone, its salts and their use as pharmaceuticals, Google Patents.
- 34.Sun Y, Zhang G, Zhang Z, Yu P, Zhong H et al. (2012) Novel multi-functional nitrones for treatment of ischemic stroke. , Bioorganic and medicinal chemistry 20, 3939-3945.
- 35.Zhang Z J, Cheang L C, Wang M W, Lee S M. (2011) Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. , Int J Mol Med 27, 195-203.
- 36.Wang B, Shravah J, Luo H, Raedschelders K, Chen D D et al. (2009) Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. , Biochem Biophys Res Commun 389, 105-111.
- 37.K M Robinson, M S Janes, Pehar M, J S Monette, M F Ross et al. (2006) Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proceedings of the National Academy of Sciences of the United States of zAmerica 103 15038-15043.
- 38.C M Maragos, Morley D, D A Wink, T M Dunams, J E Saavedra et al. (1991) Complexes of.NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. , Journal of Medicinal Chemistry 34, 3242-3247.
- 39.L K Keefer, R W Nims, K M Davies, D A Wink. (1996) NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms, Methods in enzymology 268. 281-293.
- 40.Feelisch M. (1998) The use of nitric oxide donors in pharmacological studies. , Naunyn-Schmiedeberg’s Archives of Pharmacology 358, 113-122.
- 41.Ischiropoulos H, Duran D, Horwitz J. (1995) inhibition of DOPA synthesis in PC12 cells,Journal of neurochemistry65,2366-2372ute ischemic stroke,The New England journal of medicine 354. 588-600.
- 43.Kojima H, Sakurai K, Kikuchi K, Kawahara S, Kirino Y et al. (1998) Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore, Chemical and pharmaceutical bulletin 46. 373-375.
- 44.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y et al. (1998) Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. , Analytical chemistry 70, 2446-2453.
- 45.Ischiropoulos H, Gow A, S R Thom, N W Kooy, J A Royall et al. (1999) Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. , Methods Enzymol 301, 367-373.
- 46.Jourd’heuil D, F L Jourd’heuil, P S Kutchukian, R A Musah, D A Wink et al. (2001) Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. , The Journal of biological chemistry 276, 28799-28805.
- 47.K R Maples, Ma F, Y K Zhang. (2001) Comparison of the radical trapping ability of PBN. , S-PBN and NXY-059, Free Radical Research 34, 417-426.
- 48.Watanabe T, Tahara M, Todo S. (2008) The novel antioxidant edaravone: from bench to bedside. , Cardiovasc Ther 26, 101-114.
- 49.C A Lipinski, Lombardo F, B W Dominy, P J Feeney. (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1PII of original article: S0169-409X(96)00423-1. The article was originally published in Advanced Drug Delivery Reviews,23(1997),3–25.1,Advanced Drug Delivery Reviews. 46, 3-26.
- 50.C A Lipinski. (2004) Lead- and drug-like compounds: the rule-of-five revolution, Drug Discovery Today:. , Technologies 1, 337-341.
- 51.C X Wang, Shuaib A. (2004) NXY-059: a neuroprotective agent in acute stroke. , International journal of clinical practice 58, 964-969.
- 52.H C Diener, K R Lees, Lyden P, Grotta J, Davalos A et al. (2008) NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials, Stroke; a journal of cerebral circulation. 39, 1751-1758.
Cited by (7)
- 1.Sun Haiying, Zhang Lin, Shen Dan, 2017, Urantide protects CCl4-induced liver injury via inhibiting GPR14 signal in mice, Biotechnology & Biotechnological Equipment, 31(1), 156, 10.1080/13102818.2016.1253436
- 2.Yin Jie, Wu Miaomiao, Li Yuying, Ren Wenkai, Xiao Hao, et al, 2017, Toxicity assessment of hydrogen peroxide on Toll-like receptor system, apoptosis, and mitochondrial respiration in piglets and IPEC-J2 cells, Oncotarget, 8(2), 3124, 10.18632/oncotarget.13844
- 3.Qi Tianjie, Li Haitao, Li Shuai, 2017, Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice, Oncotarget, 8(22), 36658, 10.18632/oncotarget.17560
- 4.Wu Guojun, Zhou Wenhong, Zhao Junfeng, Pan Xiaohua, Sun Yongjie, et al, 2017, Matrine alleviates lipopolysaccharide-induced intestinal inflammation and oxidative stress via CCR7 signal, Oncotarget, 8(7), 11621, 10.18632/oncotarget.14598
- 5.Yang Hongfu, Sun Rongqing, Ma Ning, Liu Qilong, Sun Xiaoge, et al, 2017, Inhibition of nuclear factor-κB signal by pyrrolidine dithiocarbamate alleviates lipopolysaccharide-induced acute lung injury, Oncotarget, 8(29), 47296, 10.18632/oncotarget.17624
- 6.Yin Jie, Li Yuying, Han Hui, Zheng Jie, Wang Lijian, et al, 2017, Effects of Lysine deficiency and Lys-Lys dipeptide on cellular apoptosis and amino acids metabolism, Molecular Nutrition & Food Research, 61(9), 1600754, 10.1002/mnfr.201600754
- 7.Wang Bin, Li Yansen, Mizu Masami, Furuta Toma, Li ChunMei, 2017, Protective effect of sugar cane extract against dextran sulfate sodium-induced colonic inflammation in mice, Tissue and Cell, 49(1), 8, 10.1016/j.tice.2016.12.008
