Abstract
The current uncontrollable outbreak of novel coronavirus (COVID-19) has unleashed severe global consequences in all aspects of life and society, bringing the whole world to a complete halt and has modeled significant threats to the global economy. The COVID-19 infection manifests with flu-like symptoms such as cough, cold, and fever resulting in acute respiratory distress syndrome (ARDS), lung dysfunction, and other systemic complications in critical patients are creating panic across the globe. However, the licensed vaccine has started to show up; some resulted in side effects that would limit its possibility in some circumstances as allergic personnel, for example. Moreover, the production and approval of new drugs is a very complicated process and takes a long time. On the other hand, stem cells have gone the extra mile and intensively investigated at preclinical and clinical studies in various degenerative diseases, including infectious ones. Stem cells are proposed as a broad-spectrum therapeutic agent, which may suppress the exaggerated immune response and promote endogenous repair by enhancing COVID-19 infected lung microenvironment. Also, stem cells have different application manners, either direct transplantation, exosome transplantation, or drug delivery of specific cytokines or nanoparticles with antiviral property by engineering stem cells. This review discusses and summarizes the possible emerging role of cell-based therapy, especially stem cell therapy, as an alternative promising therapeutic option for the treatment and control of novel COVID-19 and its potential role in tissue rejuvenation after COVID-19 infection.
Author Contributions
Academic Editor: Jose Luis Turabian, Health Center Santa Maria de Benquerencia Toledo, Spain.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Hoda Elkhenany, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The recent uncontrollable outbreak of novel coronavirus (COVID-19) infection is wreaking havoc in several countries. COVID-19 has first reported in Wuhan State of Hubei Province in China, is now a severe novel threat to public health with the emergence world over 1. On Dec. 12, 2019, the first patient of pneumonia was observed in Wuhan, China. After then cluster cases of severe pneumonia epidemic of unknown origin were again recorded in Wuhan city, China. At the end of December 2019, these cases have quickly been transmitted in China and many other countries worldwide 2. On Mar. 11, 2020, The World Health Organization (WHO) has declared; COVID-19 is a pandemic and has modeled severe public health threats and the global economy 3, 4.
The COVID-19 disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), an aggressive strain and highly infectious pathogen that mainly targets the human upper respiratory system 5, 6. Genomic sequence data of SARS-COV-2 revealed that COVID-19 is highly similar to bat SARS-like coronavirus (bat CoV RaTG13) (96.2%) and pangolin SARS-like coronavirus (86%-92%). Based on these analyses, bats could be the possible primary host for this virus 7, 8, 9. To date, there is doubt about the efficacy of approved vaccine. Therefore, there is an extremely urgent global priority to identify therapeutic options as soon as possible to prevent and control the pandemic.Due to the lack of effective and specific treatment regimen against novel coronavirus and considering the potential menace of this pandemic, researchers have been battling to understand the molecular biology of this novel strain and disease pathophysiology to uncover valuable therapeutic modalities and discover effective drug treatment against the virus.
In the meantime, there is a superhero therapeutic compartment that has proven to be efficient in a wide range of degenerative diseases, which is stem cells. It also raised as an alternative therapeutic agent because till now; there is no logical explanation of why some patients are more drastically affected by COVID-19 infection. However, the majority would recover very fast. We do believe that endogenous stem cells may play a role in such different responses.
Since COVID-19 infection is mainly detected in the upper and lower respiratory tracts but not in the spleen, bone marrow, lymph nodes, and heart. Patients with COVID-19 infection showed various significant clinicopathological changes in their lower respiratory tract, immune organs, and systemic blood vessels, emphasizing that viral infection may modulate and over-activate immune responses in the human body. However, immune modulation approaches might be potentially helpful to improve antiviral immunity and reduce the viral load, enhance outcomes and recovery of COVID-19 patients. In this regard, stem cell-based therapy is a novel emerging potential intervention that may help inhibit the overreaction of immune response and promote endogenous repair by enhancing the SARS-COV-2 infected lung microenvironment. This review discusses and summarizes the possible emerging role of stem cell therapy as an alternative therapeutic option for the treatment and control of novel COVID-19.
Methods/Source of Data
We searched the relevant published literature on PubMed, Embase, Google Scholar, Medline, Science Direct, WHO and disease Control and Prevention (CDC) online publication databases and was selected using the following keywords and phrases Stem cells, engineered stem cells, Exosome, Lung fibrosis, Lung regeneration, COVID-19 and SARS-COV-1 and 2. A literature search was conducted from April 2020. We also used the following website to retrieve the registered clinical trials (https://www.clinicaltrials.gov/). Literature type include online published international peer-reviewed articles, commentaries, online reports, editorials and electronic books for this review article.
Results
Novel COVID-19 Pathogenesis and Clinical Presentation
Novel coronavirus (corona = crown-like spikes) small enveloped, positive-sense single-stranded RNA (+ssRNA) virus belongs to the family Coronaviridae. The virus is transmitted predominantly through direct close contact with an infected person; small respiratory droplets and aerosolization/fecal-oral route are also strongly possible. Infection is more contagious when patients are symptomatic, but it can also be transmitted with asymptomatic patients' close contact and before symptoms appear 10. The infected droplets of COVID-19 can transfer up to 1-2 meters. The novel coronavirus can survive for up to 3 hours in aerosols and 72 hours on hard and nonporous surfaces 11. The onset of SARS-CoV-2 symptoms appears in 5.2 days, with a median incubation period of 14 days. The incubation time is dependent on various factors such as the patient's age, immunity, and history of acute and chronic diseases, but 97.5% of patients can develop symptoms in 11.5 days after infection 12. COVID-19 infection is susceptible to all ages, but the vulnerable population is more sensitive.
Patients with chronic diseases (diabetes, asthma, cardiovascular disease, kidney failure, obesity, malignancy, etc.) and older individuals have an increased risk of disease severity and fatality. Recent studies have identified human angiotensin-converting enzyme 2 (ACE2) as a crucial receptor for the SARS-CoV-2 and can enter the host cell's respiratory mucosa through the ACE2 receptor 13, 14. ACE2 enzyme is the most abundant enzyme in the lung's alveolar cells and is expressed in the nasal mucosa, esophagus, bronchus, gastrointestinal tract, kidney, ileum, and bladder. Viral replication primarily occurs in the upper respiratory tract's mucosal epithelium and later, with further multiplication, causes a severe lower respiratory tract infection 15. It has been identified recently that coronavirus spike protein (glycoprotein) has a strong binding affinity to human ACE2 receptor, and both SARS-COV and SARS-COV-2 spike proteins have a high degree of homology and share 76.5% identity in amino acid sequences 14, 16. The spike proteins of SARS-COV and SARS-COV-2 viruses help the attachment and entry of the virus into the host cells (Figure 1). It has suggested that attachment of the SARS-CoV-2 spike protein to the ACE-2 receptor and the complex transmembrane protease, serine 2 (TMPRSS2- crucial for entry of virus) on the surface of the cell membrane leading to cleavage of ACE2 receptor and open the spike protein, therefore, enabling viral entry into the target cell. After entry of the virus, the viral ssRNA unveiled in the host cell. After the virus enters the host cell, the viral genome is transcribed, and viral proteins are synthesized. After ACE2 receptor binding and fusion, virus-specific RNA and proteins are synthesized in the cytoplasm. Viral nucleocapsids assembled at the cell membrane, and genomic RNA in the cytoplasm is incorporated as the mature particle forms by budding into the lumen of the endoplasmic reticulum 17, 18. The mature virions release genetic material into the cell, imposing the cell to make copies of the virus that are transmitted to infect other cells (Figure 1).
Figure 1.SARS-CoV-2 transmission, entry life cycle in host cells. Spike (S) proteins of SARS-CoV-2 are attached to receptors (ACE2) on the target cell membrane's surface through the endosomal pathway. After entry of the virus, the viral ssRNA unveiled in the host cell. After ACE2 receptor binding and fusion, virus-specific RNA and proteins are synthesized in the cytoplasm. After the virus's entry into the host cell, the viral genome is transcribed, and viral proteins are synthesized. Viral nucleocapsids, assembled at the cell membrane and genomic RNA, are incorporated as mature particle forms by budding into the endoplasmic reticulum's lumen. The mature virions are then released through the exocytosis process.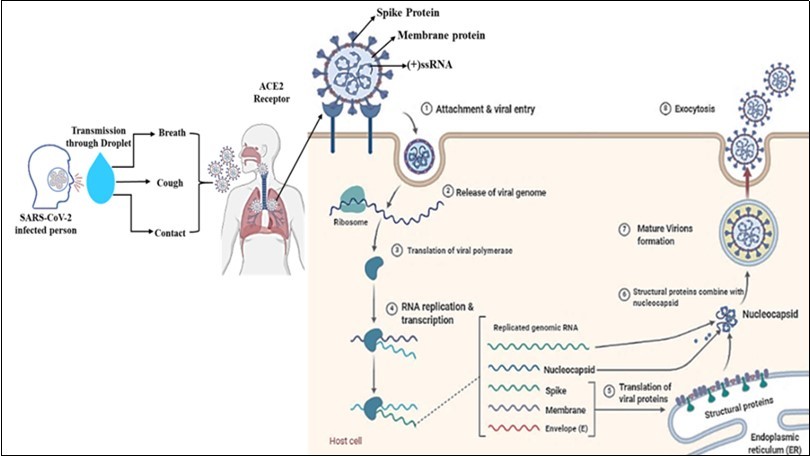
Initially, the majority of COVID-19+ve patients either asymptomatic or showed usual flu-like symptoms such as temperature (98%), dry cough (76%), dyspnoea (55%), and fatigue (44%). Remarkably, few patients faced difficulty breathing, chest pain, difficulty walking, weakness, reduced lymphocyte counts, and other uncommon clinical features like runny nose, sore throat, nasal congestion, sputum production, headache, vomiting, dyspnoea, diarrhea, and anosmia 19. The global case fatality rate (CFR) across all communities is about 4.9% 20.
Mesenchymal Stem Cells (MSCs)
MSCs are naïve cells with no identity but can differentiate into more specialized cells such as cartilage and bone cells 21. As defined by the "International Society of Cell Therapy (ISCT)," MSCs should meet specific criteria to be considered as real stem cells 22. These criteria are 1- the ability to adhere to plastics with fibroblastic shape, 2- positively express specific surface markers as "CD105, CD73 and CD90" but not "CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR" and 3- capable of differentiating into bone cell "osteoblast", fat cell "adipocyte" and cartilage cell "chondrocyte" in the presence of appropriate growth factors for each cell line 23.
MSCs have proven to restore lung function in various viral affections shares the same symptoms with COVID-19. For instance, BMSCs have been demonstrated to efficiently promote rehabilitation, survival rates and mitigate the inflammatory response in animal models infected with influenza A H1N1, H5N1, and H9N2 avian influenza 24, 25, 26. Also, Umbilical cord mesenchymal stem cells (UCMSCs) restored alveolar fluid clearance and membrane permeability after influenza A virus H5N1 27
Similarly, stem cells strongly proposed to be involved in the treatment protocol strategy of COVID-19. They have a high potential to modulate the immune system (decrease the hyperimmune activity) and stimulate the damaged tissues' regenerative process, hence accelerating recovery. Moreover, Stem cells isolated from the umbilical cord, adipose tissue, and placenta have been proven resistant to SARS-Cov-2 infection as confirmed by low ACE2 expression 28.
Additionally, stem cells have been demonstrated to shed tiny fragments at a nano-size range named later as exosomes. These particles are composed of a phospholipid membrane, which envelops proteins, cytokines, and genetic messengers. The function of released exosomes is mainly to help stem cells to work remotely at peripheral sites in the body by delivering their regenerative cues.
At the clinical application, exosomes are believed to be more advantageous as a therapeutic agent than stem cells. It will fulfill the demanded regenerative effect entirely similar to their parent cells. Moreover, exosomes provide hope for easy, safe, affordable, and off-the-shelf biological therapy 29, 30, 31, 32, 33. Also, it is worth to mention that exosome derived from MSCs (EX-MSCs) has tested for its protection as well as therapeutic potential in some viral diseases as Hepatitis C Virus (HCV), Herpes Virus (HSV), and influenza virus at in vitro and in vivo 34, 35, 36. Moreover, exosomes can play a significant role in rejuvenating degenerated lung tissues, secondary to SARS-CoV-2, as it possesses antioxidant, antifibrotic, anti-inflammatory, anti-apoptotic, matrix integrity, and other regeneration cues 37.
The Expected Outcome from Stem Cells and its Derivatives Transplantation to COVID 19 Patients
Treatment of viral disease could be approached by either preventing the virus from entering the cells, restrict virus survival and replication in the hosting cells, and inhibit excessive host immune response 38.
Many studies consensus that stem cells harbor a potential antiviral effect against polyomavirus, influenza A, and cytomegalovirus 27, 39, 40. Moreover, Stem cells have a strong regenerative and therapeutic effect as they can produce cytokines capable of stimulating cell proliferation and differentiation, enhancing vascularization, and reducing fibrosis (Figure 2).
Figure 2.The expected outcome from stem cells and its derivatives transplantation to COVID 19 patients.
Homing
Although stem cells can migrate to the site of injury in various organs upon intravenous (IV) transplantation, there is a piece of high evidence that stem cells got entrapped in the lung after IV transplantation 41, 42. This spontaneous homing capacity of stem cells to the lung will significantly benefit the lung regeneration procedure. It's also worth mentioning that entrapment of MSCs in the lung resulted in its activation and enhanced the secretion of TSG-6 (anti-inflammatory protein) 43.
Antiviral Effect
Swartzendruber and his group showed that the pluripotent stem cells exhibit antiviral potential and suppress polyomavirus function counter to differentiated somatic cells, which were more susceptible to the infection 39, 44. This finding proposed that stem cells may possess a defense mechanism by which it can degrade the foreign DNA 39. A lot of studies have conducted to prove the efficacy of MSCs and their derivatives as therapeutic agents in viral infections from then onwards.
UCMSCs have been demonstrated to restore the alveolar epithelial cells permeability and alveolar fluid clearance related to Influenza A (H5N1) virus infection in the rat animal model 27. Also, BMSCs-conditioned media has shown productive antiviral activity against HSV in vitro as it contains IL-6 and TNF-α 36. This finding confirmed by an in vivo study in which BMSCs injection resulted in a 70% survival rate compared to 10% in the control group. These results attributed to the ability of BMSCs to activate T cell proliferation, reduce the pro-inflammatory cytokines IL-6 and TNF-α, and enhance INF-γ production.
Similarly, EX-MSCs can provide a potential antiviral function. For instance, EX-UCMSCs have been demonstrated to suppress HCV replication in vitro 34. This antiviral effect is attributed to a specific miRNA mixture (let-7f, miR-145, miR-199a, and miR-221) released by EX-UCMSCs. This finding suggests the EX-UCMSCs as an optimal adjuvant of anti-HCV therapy. Furthermore, extracellular vesicles derived from swine BMSCs (EV-BMSCs) exhibited anti-influenza viral activity in vitro by inhibiting the virus replication and reducing the virus-related lung epithelial cells apoptotic effect 35. At the in vivo pig model, intra-tracheal administration of EV-BMSCs could successfully provide antiviral and anti-inflammatory effects that alleviate the influenza virus (H1N1) related lung lesions.
Immunomodulatory and Anti-Inflammatory Effect
It has demonstrated that the high fatality rate of COVID-19 hails from the acute hyper-immune response from the lung immune system (or hyperactivity of the lung immune system); this phenomenon is known as ARDS 45, 46.
In 2015, two patients suffering from ARDS succumbed BMSCs transplantation. The treatment was very efficient in resolving the inflammation and suppressing T-cell responses, and induction of regulatory phenotypes in T cells, monocytes, and neutrophils 47. Similarly, after allergic asthma, the regeneration of lung tissues was also obtained after intra-tracheal administration of BMSCs in mice model.
Since the administration of IFN-α was influential in the treatment of COVID-19 patients and resulted in a high viral mRNA elimination rate 48, engineered stem cells designed to deliver INF-α may provide a successful strategy in the treatment of COVID-19. IFN-α-engineered MSCs has tested before for its efficacy to control tumor in a mouse "plasmacytoma model" 49 and acute myeloid leukemia 50.
EX-MSCs have shown the same anti-inflammatory and immunological response as their parent cells. For example, EX-ADSCs were reported to reduce IFN-γ, consequently inhibiting T cell activation and induced macrophage polarization 51, 52. Recently, exosomes derived from placenta MSCs (EX-PLMSCs) predominantly reduced TNF-α, IL-1β, IL-12, and IFN-γ inflammatory cytokines and increased the level of IL-10 immunosuppressive cytokine; hence it resulted in suppressing inflammation 53.
Modulation of Lung Fibrosis and Endothelial Barrier Permeability
Lung fibrosis is one of the most severe complications of COVID-19. Fibrosis results in tissue thickening (as confirmed by histopathological examination), which hinders gas exchange and hence lower blood oxygen tension in which patients manifest ARDS symptoms 54, 55. Stem cells and their derivatives have provided an innovative therapeutic approach to resolve the associated COVID-19 lung fibrosis.
Intravenous injection of UC-MSCs and BMSCs have been shown to effectively ameliorate lung fibrosis in different animal models 56, 57. Moreover, stem cells and their conditioning media exhibited a high potential to enhance the endothelial barrier permeability and restore the alveolar fluid clearance capacity (which was disturbed by E. coli endotoxin) through keratinocyte growth factor (KGF) 58. Interestingly, intrapulmonary administration of BMSCs had a similar effect in that it could restore the alveolar permeability and reduce pulmonary edema 59. Moreover, Asmussen et al. added that MSCs dose plays an essential role in the clinical output. A dose of 10×106 BMSCs/kg reduced the pulmonary edema more efficiently than 5×106 cells/kg in the E. coli pneumonia sheep model 60.
In order to enhance the alveolar epithelial cell regeneration, which eventually will impact pulmonary permeability dominance, Zhang et al. have proposed to engineer BMSCs to overexpress p130 or E2F4 61. The results of this study revealed that p130/E2F4 could inhibit lung fibrosis, promote MSCs differentiation into epithelial cells, and hence enhanced alveolar epithelial permeability.
ACE2 has been reported to prevent lung injury resulting from septicemia, acid inhalation, or endotoxin shock 62. Also, it showed a protective effect on pulmonary endothelial cells from apoptosis in vivo and in vitro 63. The exploitation of stem cells to deliver the ACE2 gene in what is known as engineered stem cells has been proposed to treat lung injury effectively. For example, ACE2 engineered UC-MSCs significantly alleviated inflammation, fibrosis, and pulmonary permeability 64, 65. It also has shown a protective effect on DNA damage and possesses antioxidant property 66, 67. Likewise, ACE2 engineered BMSCs manifested an anti-inflammatory effect and improved the pulmonary endothelial function 66.
Applying this hypothesis based on the preclinical findings in Corona virus-infected patients may be tricky and not guaranteed. The reason lies in the possibility of ACE2-cells to get infected with CoV-19, similar to SARS-2003 68, 69, 70. These last findings spur Whuan medical staff to use ACE2–ve MSCs in their treatment protocol strategy in Beijing YouAn Hospital 71.
Anti-Apoptotic and Antioxidant Effect
Stem cells have a potential role in preventing cell death. For instance, BMSCs has shown a significant effect on Isoproterenol-induced lung injury (ISP-LI) as it reduced the caspase-3 activity and upregulated nuclear-related factor-2 (Nrf2, antioxidant marker) 72.
EX-ADSCs exhibit a protective effect on cardiomyocytes against reactive oxygen species (ROS), which has a drastic impact on cell viability 73. In terms of the engineering of stem cells to meet specific criteria as alleviating apoptosis, exosomes derived from GATA-4 engineered MSCs have proven to protect cells in damaged tissues upon its transplantation and increase survival rate 74. Furthermore, EX-BMSCs could alleviate lung ischemia by delivering "MiR-21-5p" anti-apoptotic miRNA and protecting cells from oxidative stress 75.
MSCs also were reported to have a potent antioxidant effect. BMSCs reported reducing ROS production in HCL injured lung animal model through upregulation of antioxidant enzyme Hemeoxygenase-1 (HO-1) 76. Interestingly, apoptotic ADSCs (serum-deprived cells) have been shown to produce a superior antioxidant protective effect on lung and kidney injury than healthy ones 77. This effect was attributed to high antioxidant protein expression as HO-1, glucocorticoid (GR), and NAD(P)H dehydrogenase (NQO-1). Also, EX-UCMSCs exhibited a protective antioxidant effect on UV-treated cells as detected by high antioxidant proteins, "glutathione peroxidase 1 (GPX-1), superoxide dismutase (SOD), and catalase", expression 78.
Clinical Trials
In terms of testing stem cells efficacy from various sources (including BMSCs, ADSCs, Decidual stromal cells, Menstrual blood stem cells, and HUCMSCs) as a therapeutic agent for ARDS, which is the primary symptom of COVID-19, there were about 11 clinical trials have been registered from 2013 to 2020.
Some clinical trials has shown that stem cells are safe, tolerable, and effective in alleviating ARDS related symptoms as shown in Table 1. Currently nine clinical trials registered, aiming to investigate stem cells' efficacy in the COVID-19 treatment strategy. Most of these trials from China, one from Brazil, and one from Amman.
UC-MSCs were used successfully to treat one COVID-19 positive female patient (65-years old) in which she received three doses; each one was 50*106 every three days 62. Another clinical trial included seven patients diagnosed as COVID-19 to be treated with ACE2-ve-MSCs transplantation 71. The results of this study revealed that ACE2-ve-MSCs transplantation has significantly improved pulmonary function as well as increased the peripheral lymphocyte and IL-10 cytokines.
Table 1. Some Clinical Trials of various sources of stem cells as a therapeutic agent for ARDS.| Trials | Methodology | Results | Ref |
| Allogeneic ADSCs (1*106 cell/kg) | 12 ARDS | The treatment was safe and tolerable; but non-significant to the placebo group. | (104) |
| BMSCs(10*106 per kg) | 9 ARDS | Resulted in significantly higher clinical output. The mortality rate was 22% (other reasons than treatment protocol). | (105) |
| UC-MSCs | 9 ARDS | Reduced the inflammatory biomarkers and increased the immune T-cell markers (helper, cytotoxic, and regulatory T-cell) | (106) |
EX-MSCs have been previously tested for their potential to treat lung-related diseases as a chronic obstructive pulmonary disease (COPD) 53, 79, Asthma 80, lung fibrosis 81, 82, and ARDS 83. These studies confirmed a high potential of exosomes to reduce inflammation and contribute to tissue remodeling. Consequently, these optimistic outputs boost their incorporation in clinical trials. Recently, a clinical trial proposed to use EX-ADSCs through inhalation for the treatment of pneumonia-related COVID-19 infection (NCT04276987). Two completed studies associated with EX-MSCs and Covid-19 have been conducted in China (NCT04276987) and Russia (NCT04491240). Exosomes inhalation can diminish lung tissue inflammation and promote tissue regeneration by boosting the immune cells and promote tissue regeneration. They also validate that exosome inhalation is a safe and productive treatment 1.
Precautions of Stem Cells Transplantation
In terms of precautions that should be considered in stem cell therapy, there will be three aspects, the first aspect related to donors, the second related to cell therapy preparation, and the final one associated with patients (therapy recipients).
Donors should be screened thoroughly. Selection of the donors should be subject to some considerations as the donor age and health status as they have proven to impact the stem cells quality and the derived exosomes.
Cell therapy preparation: High precautions should be taken to prevent contaminations. Also, cells should go through many investigations to prove their viability, efficacy, and safety. Taken into consideration, the raised warnings from using an unproven stem cell therapy instead of properly tested ones are the physician's primary responsibility, as discussed by 84, 85. Furthermore, the cell expansion status (whether 2D or 3D culture) is dramatically affecting the stem cells quality, so reporting this piece of information in the medical report will significantly enhance the evaluation of the therapeutic efficacy and future trials 86, 87.
Recipients should be checked if they have a cancer history. Stem cell therapy may worsen cancer patients' health status as proved in vitro and in vivo studies 88. In this regard, we highly recommend using engineered MSCs or their derivatives exosomes because some of the patients may have subclinical cancer or tumors; this will open the door for more trustable, controllable, and safe therapeutic agents 89, 90. The administration route should be through IV as it has demonstrated that stem cells got entrapped in the lung in IV administration 91. Moreover, as reported by Atluri, Manchikanti 92, monitoring the patients during the therapy transplantation is very important to prevent organ embolization and allergic reactions. Also, as it is well known that MSCs compared to controls were associated with an increased risk of fever, controlling the body temperature is of high demand 93.
Future Directions to Control COVID-19 Transmission.
Herein, we shed light on the possible therapeutic potential effect of stem cell therapy and its derivatives on COVID-19. We will also highlight the possibility of involving genetic engineering and nanotechnology to enhance stem cells' regenerative capacity and their derivatives (Figure 3).
Figure 3.Future directions to control COVID-19 transmission. 1- Adult stem cells (the upper raw), including MSCs, have proved to be effective in promoting the recovery of COVID-19 patients. Also, as per the available data, we propose engineering MSCs either by genetic engineering in which we can direct MSCs to produce specific growth factors or loading MSCs with NPs (known to have an antiviral capacity). 2- Exosome (the lower raw), since exosome proved to have similar potential as their parent cells. Then exosomes derived from the naïve MSCs, genetically engineered MSCs, or NPs engineered MSCs can be involved in the therapeutic protocol as well with higher efficiency.
As shown in recent studies and clinical trials that incorporated stem cell therapy in the treatment protocol of some viral infection cases and COVID-19, in particular, that stem cells have a high potential to modulate the immune response and regenerate tissues. Herein, we propose a couple of ways to enhance the stem cell's therapeutic efficiency and increasing its potential. That can be accomplished by either genetic engineering of stem cells or NPs engineering. MSCs genetic engineering can be achieved by either upregulation of specific genes (which are necessary for the regeneration process) or downregulation of other genes (which may be involved in the virus virulence) 94. Engineered MSCs have proven high potential to control tumors, provide high vascularization, attenuate inflammation, and act as a vaccine by providing immune protection against some viruses 49, 95, 96, 97.
In addition, stem cells are proposed as an excellent mediator to deliver NPs with antiviral properties since they possess a high affinity to migrate into the lung. The nanotechnology field advances lead to inducing nanoparticles (NPs) modifications to accommodate the viral infection control strategy. For instance, some NPs as Rheum tanguticum, tannic acid-modified silver (TA-Ag), polyethylene glycol coated zinc oxide (ZnO-PEG), and Gold/Copper Sulfide (Au/CuS) have shown to be effective against Herpes simplex virus type 1 (HSV-1), HSV-2, influenza virus (H1N1), and Human norovirus respectively 98, 99, 100, 101.
To get a closer idea about the effect of NPs on the currently pandemic SARS-2 infection, we will spot the light on a couple of NPs that have been modified and tested on viruses from the same family (Coronavirus). The antiviral activity of Qdots has been investigated on human coronavirus HCoV-229E in which Qdots cause inhibition of the virus at the entry stage and replication stage due to the capacity of the NPs to interact with the virus entry receptors 102. Another example is using gold NPs against Middle East respiratory syndrome coronavirus (MERS-CoV) 103. In this study, authors could raise the peptide pregnancy-induced hypertension (PIH, peptide has an inhibitory effect on MERS-CoV membrane fusion to host cells) potential 10-fold by its integration with gold NPs. In the same manner, NPs can be used to target the SARS-2 virus as well.
As explained earlier, exosomes can supersede stem cells efficiently and beat on the raised issues against their use. Exosomes derived from the previously mentioned engineered MSCs can also enhance their regenerative capacity and increase the successful cure rate. Moreover, the possibility of being administered by inhalation can decrease the overload of hospitals.
In conclusion, with the absence of specific and effective antiviral treatment against novel coronavirus, stem cells and their derivatives exosomes are our great hope and efficient promising tools as antiviral agents for treating COVID-19 patients. These emerging approaches might improve critically ill pneumonia patients' outcomes through anti-inflammatory, anti-immunomodulatory actions, and promoting tissue repair and recovery.
Funding Source
None declared.
Ethical Approval
Not applicable
Acknowledgment
H.E. would like to acknowledge the Women for Africa Foundation (FMxA) for supporting her through the science by women program to meet her passion for regenerative medicine. Also, ICMR/AU-STRC funded her travel to India through Health Practitioners and Researchers Capacity Building Scheme, which allowed her to collaborate with NICPR colleagues. H.E. and S.G. would like to thank BioRender online software for their support.
References
- 1.Lu H, Stratton C W, Tang Y W. (2020) Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. , J Med Virol 92(4), 401-2.
- 2.Guan W J, Ni Z Y, Hu Y, Liang W H, Ou C Q et al. (2020) in China. Clinical Characteristics of Coronavirus Disease , N Engl J Med.
- 3.Xue K, Zhang X, Liu K. (2015) . , ISOLATION, CULTURE AND IDENTIFICATION OF CARTILAGE DERIVED STEM CELLS FROM THREE SUBTYPES OF CARTILAGES]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 29(4), 483-9.
- 4.Hass R, Kasper C, Böhm S, Jacobs R. (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 9(1), 12.
- 5.Rothan H A, Byrareddy S N. (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. , J Autoimmun 102433.
- 6.Gupta S, Elkhenany H, Kumar P, Okda F A. (2020) Animals in the COVID-19 Era: Between Being a source, Victims, or Maybe our Hope to Overcome it!. , INTERNATIONAL JOURNAL OF CORONAVIRUSES 1(4), 12-25.
- 7.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. , Nature 579(7798), 270-3.
- 8.Lu R, Zhao X, Li J, Niu P, Yang B et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 395(10224), 565-74.
- 9.Wan Y, Shang J, Graham R, Baric R S, Li F. (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. , J 94(7).
- 10.WHO. (2020) Situation reports. Available from:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 11.N van Doremalen, Bushmaker T, Morris D H, Holbrook M G, Gamble A et al. (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. , New Engl
- 12.Lauer S A, Grantz K H, Bi Q, Jones F K, Zheng Q et al. (2020) The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of internal medicine.
- 13.Letko M, Marzi A, Munster V. (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. , Nature microbiology 5(4), 562-9.
- 14.Xu X, Chen P, Wang J, Feng J, Zhou H et al. (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China Life Sciences. 63(3), 457-60.
- 15.Zou L, Ruan F, Huang M, Liang L, Huang H et al. (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. , New Engl J Med 382(12), 1177-9.
- 16.Li F, Li W, Farzan M, Harrison S C. (2005) Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. , Science 309(5742), 1864-8.
- 17.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L et al. (2020) Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. , BioRxiv
- 18.Shereen M A, Khan S, Kazmi A, Bashir N, Siddique R. (2020) COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. , J Adv Res 24, 91-8.
- 19.Huang C, Wang Y, Li X, Ren L, Zhao J et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. , China, The Lancet 395(10223), 497-506.
- 20.Florindo H F, Kleiner R, Vaskovich-Koubi D, Acúrcio R C, Carreira B et al. (2020) Immune-mediated approaches against COVID-19. Nature nanotechnology. 15(8), 630-45.
- 22.Dominici M, K Le Blanc, Mueller I, Slaper-Cortenbach I, Marini F et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. , Cytotherapy 8(4), 315-7.
- 23.El-Derby A M, Ahmed T A, Abd El-Hameed AM, Elkhenany H, Saad S M et al. (2020) Adult Stem Cells: Mesenchymal Stromal Cells, Endothelial Progenitor Cells, and Pericytes. Regenerative Medicine and Stem Cell Biology:. 109-49.
- 24.Darwish I, Banner D, Mubareka S, Kim H, Besla R et al. (2013) Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One. 8(8), 71761.
- 25.Gotts J E, Abbott J, Matthay M A. (2014) Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. , Am J Physiol Lung Cell Mol Physiol 307(5), 395-406.
- 26.Li Y, Xu J, Shi W, Chen C, Shao Y et al. (2016) Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. , Stem Cell Res Ther 7(1), 159.
- 27.Loy H, DIT Kuok, KPY Hui, MHL Choi, Yuen W et al. (2019) Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. , J Infect Dis 219(2), 186-96.
- 28.Cao Y, Wu H, Zhai W, Wang Y, Li M et al. (2020) A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem Cell Research. 102066.
- 29.Chen T S, Arslan F, Yin Y, Tan S S, Lai R C et al. (2011) Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. , Journal of translational medicine 9(1), 47.
- 30.Yi Y W, Lee J H, Kim S-Y, Pack C-G, Ha D H et al. (2020) Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. International journal of molecular sciences. 21(2), 665.
- 31.Mendt M, Kamerkar S, Sugimoto H, McAndrews K M, Wu C C et al. (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer. , JCI Insight 3(8).
- 32.P-UC Dinh, Paudel D, Brochu H, Popowski K D, Gracieux M C et al. (2020) Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. , Nature 11(1), 1064.
- 33.Lv L, Zeng Q, Wu S, Xie H, Chen J et al. (2015) Chapter 8 - Exosome-Based Translational Nanomedicine: The Therapeutic Potential for Drug Delivery. Mesenchymal Stem Cell Derived Exosomes In: Tang Y, Dawn B, editors , Boston: 161-76.
- 34.Qian X, Xu C, Fang S, Zhao P, Wang Y et al. (2016) Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl Med. 5(9), 1190-203.
- 35.Khatri M, Richardson L A, Meulia T. (2018) Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 9(1), 17.
- 36.Klimova R R, C Momotyuk capital Ie, Demidova N A, Yarigina E I, Kushch A A. (2018) Mesenchymal stem cells enhance immune response and protect mice against lethal herpes viral infection.. Vopr Virusol. 63(6), 261-7.
- 37.Elkhenany H, Gupta S.Mesenchymal Stem Cell–Derived Exosomes and Regenerative Medicine. Role of Exosomes in Biological Communication Systems:. 141-64.
- 38.Shetty R, Ghosh A, Honavar S G, Khamar P, Sethu S. (2020) Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: Present and future. , Indian J Ophthalmol
- 39.Swartzendruber D E, Friedrich T D, Lehman J M. (1977) Resistance of teratocarcinoma stem cells to infection with simian virus 40: early events. , J Cell Physiol 93(1), 25-30.
- 40.Belzile J P, Stark T J, Yeo G W, Spector D H. (2014) Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. , J Virol 88(8), 4021-39.
- 41.Schrepfer S, Deuse T, Reichenspurner H, Fischbein M P, Robbins R C et al. (2007) Stem cell transplantation: the lung barrier. Transplant. Proc 39(2), 573-6.
- 42.Gao J, Dennis J E, Muzic R F, Lundberg M, Caplan A I. (2001) The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 169(1), 12-20.
- 43.Lee R H, Pulin A A, Seo M J, Kota D J, Ylostalo J et al. (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5(1), 54-63.
- 44.Swartzendruber D E, Lehman J M.Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. , J Cell Physiol. 1975;85(2 Pt 1, 179-87.
- 45.Zhang T, Sun L, Feng R. (2020) Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chinese journal of tuberculosis and respiratory diseases. 43, 040.
- 46.Tang X, Du R, Wang R, Cao T, Guan L et al. (2020) . Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1. Chest .
- 47.Simonson O E, Mougiakakos D, Heldring N, Bassi G, Johansson H J et al. (2015) . In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med 4(10), 1199-213.
- 48.Yuan J, Zou R, Zeng L, Kou S, Lan J et al. (2020) The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res.
- 49.Sartoris S, Mazzocco M, Tinelli M, Martini M, Mosna F et al. (2011) Efficacy assessment of interferon-alpha-engineered mesenchymal stromal cells in a mouse plasmacytoma model. Stem Cells Dev. 20(4), 709-19.
- 50.Laura D M, Hasgur S, Guess A J, Yu M, Otsuru S et al. (2019) Combination of Interferon α, Delivered By Engineered Mesenchymal Stromal Cells, and Cytarabine Limits the Development of Acute Myeloid Leukemia, Potentially Targeting Leukemic Stem Cells. American Society of Hematology. , Washington, DC;
- 51.Blazquez R, Sanchez-Margallo F M, O de la Rosa, Dalemans W, Álvarez V et al. (2014) Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Frontiers in immunology. 5, 556.
- 52.Zhao H, Shang Q, Pan Z, Bai Y, Li Z et al. (2018) Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. , Diabetes 67(2), 235-47.
- 53.Harrell C R, Miloradovic D, Sadikot R, Fellabaum C, Markovic B S et al.Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product "Exo-d-MAPPS" in Attenuation of Chronic Airway Inflammation. Anal Cell Pathol (Amst). 2020-2020.
- 54.Wang J, Wang B J, Yang J C, Wang M Y, Chen C et al.(2020).Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease2019and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi. 36(0), 006.
- 55.Xu Z, Shi L, Wang Y, Zhang J, Huang L et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine.
- 56.Moodley Y, Atienza D, Manuelpillai U, Samuel C S, Tchongue J et al. (2009) Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. , Am J Pathol 175(1), 303-13.
- 57.Ortiz L A, Dutreil M, Fattman C, Pandey A C, Torres G et al. (2007) Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America 104(26), 11002-7.
- 58.Lee J W, Fang X, Gupta N, Serikov V, Matthay M A. (2009) Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. , Proc Natl Acad Sci U S A 106(38), 16357-62.
- 59.Gupta N, Su X, Popov B, Lee J W, Serikov V et al. (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. , J Immunol 179(3), 1855-63.
- 60.Asmussen S, Ito H, Traber D L, Lee J W, Cox R A et al. (2014) Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. , Thorax 69(9), 819-25.
- 61.Zhang X, Chen J, Xue M, Tang Y, Xu J et al. (2019) Overexpressing p130/E2F4 in mesenchymal stem cells facilitates the repair of injured alveolar epithelial cells. in LPS-induced ARDS mice. Stem cell research & therapy 10(1), 74.
- 62.Oudit G Y, Kassiri Z, Patel M P, Chappell M, Butany J et al. (2007) Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 75(1), 29-39.
- 63.Ji Y, Gao F, Sun B, Hao J, Liu Z. (2015) Angiotensin-Converting Enzyme 2 Inhibits Apoptosis of Pulmonary Endothelial Cells During Acute Lung Injury Through Suppressing SMAD2 Phosphorylation. Cell Physiol Biochem. 35(6), 2203-12.
- 64.Min F, Gao F, Li Q, Liu Z. (2015) Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep. 11(4), 2387-96.
- 65.Gao F, Li Q, Hou L, Li Z, Min F et al. (2014) Mesenchymal stem cell-based angiotensin-converting enzyme 2 in treatment of acute lung injury rat induced by bleomycin. Exp Lung Res. 40(8), 392-403.
- 66.Liu F, Gao F, Li Q, Liu Z. (2014) The functional study of human umbilical cord mesenchymal stem cells harbouring angiotensin-converting enzyme 2 in rat acute lung ischemia-reperfusion injury model. , Cell Biochem Funct 32(7), 580-9.
- 67.Zhang X, Gao F, Yan Y, Ruan Z, Liu Z. (2015) Combination therapy with human umbilical cord mesenchymal stem cells and angiotensin-converting enzyme 2 is superior for the treatment of acute lung ischemia-reperfusion injury in rats. , Cell Biochem Funct 33(3), 113-20.
- 68.Kuba K, Imai Y, Rao S, Gao H, Guo F et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. , Nat Med 11(8), 875-9.
- 69.Ge X Y, Li J L, Yang X L, Chmura A A, Zhu G et al. (2013) Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. , Nature 503(7477), 535-8.
- 70.Hoffmann M, Kleine-Weber H, Krüger N, Mueller M A, Drosten C et al. (2020) The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. , BioRxiv
- 71.Leng Z, Zhu R, Hou W, Feng Y, Yang Y et al. (2020) Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and disease. 11(2), 216-28.
- 72.Kadry M O, Abdel‐Megeed R M. (2017) Bone marrow‐derived mesenchymal stem cells mitigate caspase‐3 and 8‐hydroxy proline induced via β‐adrenergic agonist in pulmonary injured rats. , J Biochem Mol Toxicol 31(8), 21913.
- 73.Liu Z, Xu Y, Wan Y, Gao J, Chu Y et al. (2019) Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell death discovery. 5(1), 1-7.
- 74.Yu B, Kim H W, Gong M, Wang J, Millard R W et al. (2015) Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International journal of cardiology. 182, 349-60.
- 75.Jw Li, Wei L, Han Z, Chen Z. (2019) Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. , Eur J Pharmacol.; 852, 68-76.
- 76.El-Metwaly S, El-Senduny F F, El-Demerdash R S, Abdel-Aziz A F. (2019) Mesenchymal stem cells alleviate hydrochloric acid-induced lung injury through suppression of inflammation, oxidative stress and apoptosis in comparison to moxifloxacin and sildenafil. , Heliyon 5(12), 02710.
- 77.Sung P H, Chang C L, Tsai T H, Chang L T, Leu S et al. (2013) Apoptotic adipose-derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. , Stem Cell Res Ther 4(6), 155.
- 78.Deng M, Yu Z, Li D, Wang X, Zhou G et al. (2020) Human umbilical cord mesenchymal stem cell-derived and dermal fibroblast-derived extracellular vesicles protect dermal fibroblasts from ultraviolet radiation-induced photoaging in vitro. , Photochem Photobiol Sci 19(3), 406-14.
- 79.Maremanda K P, Sundar I K, Rahman I. (2019) Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. , Toxicol Appl Pharmacol 385, 114788.
- 80.Du Y M, Zhuansun Y X, Chen R, Lin L, Lin Y et al. (2018) Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 363(1), 114-20.
- 81.Sun L, Zhu M, Feng W, Lin Y, Yin J et al.. Exosomal miRNA Let-7 from Menstrual Blood-Derived Endometrial Stem Cells Alleviates Pulmonary Fibrosis through Regulating Mitochondrial DNA Damage. Oxid Med Cell Longev 2019, 4506303.
- 82.Mansouri N, Willis G R, Fernandez-Gonzalez A, Reis M, Nassiri S et al. (2019) Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. , JCI Insight 4(21).
- 83.Yu Q, Wang D, Wen X, Tang X, Qi D et al. (2020) Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/Ca(2+) signaling pathway. , Am J Physiol Lung Cell Mol Physiol 318(4), 723-741.
- 84.Levine A D, Wolf L E. (2012) The roles and responsibilities of physicians in patients' decisions about unproven stem cell therapies. , J Law Med Ethics 40(1), 122-34.
- 85.Bauer G, Elsallab M, Abou-El-Enein M. (2018) Concise Review: A Comprehensive Analysis of Reported Adverse Events in Patients Receiving Unproven Stem Cell-Based Interventions. Stem cells translational medicine. 7(9), 676-85.
- 86.Potapova I A, Gaudette G R, Brink P R, Robinson R B, Rosen M R et al. (2007) Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. , Stem Cells 25(7), 1761-8.
- 87.Bartosh T J, Ylostalo J H, Mohammadipoor A, Bazhanov N, Coble K et al. (2010) Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. , Proc Natl Acad Sci U S A 107(31), 13724-9.
- 88.Chen J, Ji T, Wu D, Jiang S, Zhao J et al. (2019) Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin α5 in hepatocellular carcinoma. , Cell death & disease 10(6), 425.
- 89.Elkhenany H, Shekshek A, Abdel-Daim M, El-Badri N. (2019) Stem Cell Therapy for Hepatocellular Carcinoma: Future Perspectives.
- 90.Vakhshiteh F, Atyabi F, Ostad S N. (2019) Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. , International journal of nanomedicine 14, 2847-59.
- 91.Walczak P, Zhang J, Gilad A A, Kedziorek D A, Ruiz-Cabello J et al.Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. , Stroke 39(5), 1569-74.
- 92.Atluri S, Manchikanti L, Hirsch J A. (2020) Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 23(2), 71-83.
- 93.Thompson M, SHJ Mei, Wolfe D, Champagne J, Fergusson D et al.Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. 2020-19.
- 94.Nowakowski A, Andrzejewska A, Janowski M, Walczak P, Lukomska B. (2013) Genetic engineering of stem cells for enhanced therapy. , Acta Neurobiol Exp (Wars) 73(1), 1-18.
- 95.Braid L R, Hu W G, Davies J E, Nagata L P. (2016) Engineered Mesenchymal Cells Improve Passive Immune Protection Against Lethal Venezuelan Equine Encephalitis Virus Exposure. Stem Cells Transl Med. 5(8), 1026-35.
- 96.Piao W, Wang H, Inoue M, Hasegawa M, Hamada H et al. (2010) Transplantation of Sendai viral angiopoietin-1-modified mesenchymal stem cells for ischemic limb disease. , Angiogenesis 13(3), 203-10.
- 97.Bao C, Guo J, Lin G, Hu M, Hu Z. (2008) TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. , Scand Cardiovasc J 42(1), 56-62.
- 98.Shen M-X, Ma N, Li M-K, Liu Y-Y, Chen T et al. (2019) . Antiviral Properties of R. tanguticum Nanoparticles on Herpes Simplex Virus Type I In Vitro and In Vivo. Frontiers in pharmacology.; 10, 959.
- 99.Orlowski P, Tomaszewska E, Gniadek M, Baska P, Nowakowska J et al. (2014) Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. , PloS one 9(8), 104-113.
- 100.Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F et al. (2019) Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. , J Biomed Sci 26(1), 70.
- 101.Broglie J J, Alston B, Yang C, Ma L, Adcock A F et al. (2015) Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-Like Particles. PloS one. 10-10.
- 102.Łoczechin A, Séron K, Barras A, Giovanelli E, Belouzard S et al. (2019) Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. , ACS Applied Materials & Interfaces 11(46), 42964-74.
- 103.Huang X, Li M, Xu Y, Zhang J, Meng X et al. (2019) Novel Gold Nanorod-Based HR1 Peptide Inhibitor for Middle East Respiratory Syndrome Coronavirus. , ACS Applied Materials & Interfaces 11(22), 19799-807.
- 104.Zheng G, Huang L, Tong H, Shu Q, Hu Y et al. (2014) Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. , Respir Res 15, 39.