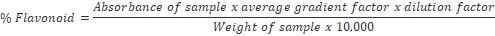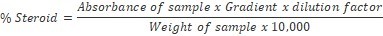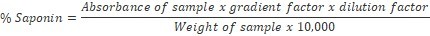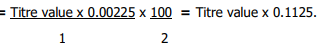Abstract
Spices have been frequently added to foods since ancient times, not only to enhance the taste but also as preservatives and medicinal agents. Their usage may be of concern due to possible contamination during processing and handling. The aim of this study was to investigate the physicochemical properties and heavy metals concentrations in some indigenous spices sold at two main markets namely Bodija and Apata markets in Ibadan, Oyo State, Nigeria. A total of eight commonly consumed spices were purposely analyzed for their proximate and mineral composition, physicochemical properties and anti-nutrients contents. Proximate analyses showed that the spices to contained (fresh matter ‘As consumed’) moisture content ranging from 11.74g in thyme to 59.36g/100g in scent leaves. Crude protein, fat, fibre, ash and carbohydrate contents ranged between 3.72 – 15.07g, 1.31 – 8.28g, 1.96 – 11.38g, 1.11 – 7.81g, and 17.80 – 50.77g/100g sample, respectively. All the spices contained high levels of potassium (176.3 – 739.6 mg), sodium (60.6 – 317 mg), calcium (78.5 – 423.9 mg), magnesium (82 – 322.1 mg) and iron (5.78 – 20.10 mg), but low levels of heavy metals – copper (0.17 – 0.68 mg), and manganese (0.32 – 1.05 mg)/100g respectively. Flavonoid was the most abundant phytochemical, while terpene was the least phytochemical in all the samples. The samples had very low concentrations of anti-nutrients, and could pose no threat to human health, as their values were within the regulatory standard. The antioxidants and phytochemicals in the spices can help in building up immunity and prevention of non-communicable diseases, hence, their consumption should be encouraged.
Author Contributions
Academic Editor: Sharif Hasan Siddiqui, Chonbuk National University, Korea.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Adeola Yewande BAMIGBOYE, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflicts of interest to declare.
Citation:
Introduction
Spices are dried parts of plants, which have been used as dietary components often to improve colour, aroma, palatability and acceptability of food, as well as influence digestion and metabolism1. They are either used in the form of dried seed, fruit, root, bark, or as vegetables2. Spices have been used for centuries by many cultures to enhance flavour and aroma, and as preservative and medicinal agents3. In Nigeria, some spices are used in the preparation of certain soups which are delicacies, and also recommended for rapid relief of ailments such as cold, malaria fever, and so on. Therapeutically, spices have been useful in the management of stomach ache, leprosy, cough, loss of appetite, rheumatoid pain, convulsion and inflammation4.
Spices have been recognized to have some medicinal properties due to their antioxidant and antimicrobial actions5. These benefits from spices are as a result of the presence of phytochemicals6,7, which make each spice to have a unique aroma and flavour. The phytochemicals present in plants are responsible for prevention of disease and promotion of health. The bioactive constituents are steroids, terpenoids, carotenoids, flavonoids, alkaloids, tannins and glycosides8. Even though spices have many benefits, they can also contain some toxic chemicals derived from the environment of their production, processing, and storage conditions. Addition of spices that may be contaminated with trace and heavy metals to food as a habit may result in accumulation of these metals in human organs and lead to different health issues both in the middle and long terms9.
The average amount of metals found in a spice can vary from spice to spice or place of production1. Natural food spices such as pepper have been reported to contain significant quantities of some trace metals10. The powder of pepper (red), turmeric and coriander spices from the local markets are usually processed by individual sellers, and to attracts customers, some of them add colourants which may contain contaminants in form of some trace metals. Addition of spices that are contaminated with trace and heavy metals above the permissible level can affect human health.
The presence of essential metals such as copper, zinc, nickel and iron in spices are useful for the healthy growth of the body; and they play vital roles as structural and functional components of metalloproteins and enzymes in living cells11; though they seem intolerable at very high levels. Due to consumption of significant amount of spices through diets, it is important to know the metal contents in these spices. This study was aimed at investigating the physico-chemical properties and heavy metals concentrations in some indigenous spices sold at Bodija and Apata markets in Ibadan, Oyo State, Nigeria. Figure 1.
Materials and Methods
Sample Collection and Preparation
A total of eight spices were purposively bought from the two markets and these spices were cameroon pepper (Capsicum chinense Linn); nutmeg (Myristicafragrans); clove (Syzgiumaromaticum); ginger (Zingiberofficinale); turmeric (Curcuma longa Linn); thyme (Thymus vulgaris L.); scent leaves (Occimumgratisimum) and garlic (Allum sativum) respectively. Samples were oven dried at 800C for 12 hrs to remove inherent water molecules; the dried materials were pulverized to form powder. Each sample was placed in an airtight container, labeled and kept at room temperature until when needed for further analyses.
Proximate Analyses
Moisture content of the samples was determined by air oven at 1050C (plus 11 Sanyo Gallenkamp PLC UK) for 4 hours. The crude protein of the samples was determined using micro-kjeldahl method12 and amount of crude protein calculated using the conversion factor of 6.25. Crude lipid was determined by weighing 5 g of dried sample into fat free extraction thimble and plugging lightly with cotton wool. The thimble was placed in the Soxhlet extractor fitted up with reflux condenser. The dried sample was extracted with petroleum ether and the crude lipid estimated as g/100g dry weight of sample and then converted to g/100g fresh sample weight. The ash content was determined by weighing 5g of sample and heated in muffle furnace (Gallenkamp, size 3) at 5500C for 4 hrs and ash calculated as g/100g original sample12. Total carbohydrate content was obtained by difference.
Mineral Analysis
Potassium and sodium content of the samples were determined by digesting the ash of the samples with perchloric acid and nitric acid, and then taking the readings on the Jenway digital flame photometer/spectronic2012. Phosphorus was determined by vanado-molybdate colorimetric method. Calcium, magnesium, iron, zinc, manganese and copper were determined spectrophotometrically by using Buck 200 atomic absorption spectrophotometer (Bulk Scientific, Norwalk) and absorption values of the samples’ minerals were compared with absorption of standards of these minerals12.
Phytochemical Screening
Flavonoid Determination
The samples were grinded, and 0.50 g of finely ground sample was weighed into a 100 ml beaker and 80 ml of 95% ethanol was added and stirred with a glass rod to prevent lumping. The mixture was filtered through a Whatman No.1 filter into a 100 ml volumetric flask and made up to mark with ethanol, and 1ml of the extract pipetted into 50 ml volumetric flask. Four drops of concentrated HCl were added, after which 0.5 g of magnesium turnings was added to develop a magenta red coloration. Standard flavonoid solutions of range 0-5 ppm were prepared and treated in a similar way with HCl and magnesium turnings like sample. The absorbance of sample and standard solutions was read on a digital Jenway V6300 Spectrophotometer at 520 nm13. The percentage flavonoid was calculated using the formula:
Glycoside Determination
Ten millilitres of extract was pipette into a 250 ml conical flask, 50 ml of chloroform was added and shaken on a Vortex Mixer for 1 hr. The mixture was filtered, 10 ml pyridine and 2 ml of 2% sodium nitroprusside were added, shaken thoroughly for 10 mins and 3 ml of 20% NaOH added to develop a brownish yellow colour. Standard solutions of glycoside ranging from 0 – 5 mg/ml were prepared and treated as the sample. The absorbance of the sample and standard solutions was read on a Spectronic21D digital Spectrophotometer at 510 nm14. The percentage of the glucoside was calculated using the formula:
Steroid Determination
About 0.5 g of sample was weighed into 100 ml beaker and 20 ml of chloroform-methanol (2:1) mixture added and shaken for 30 min on a shaker. The mixture was filtered through a Whatman No.1filter paper and resultant residue repeatedly treated with chloroform-methanol mixture until free of steroids. One millilitre (1 ml) of the filtrate was pipetted into a 30 ml test tube and 5 ml of alcoholic KOH added and shaken thoroughly. The mixture was later placed in a water bath set at 370C - 400C for 90 mins, cooled to room temperature, then 10 ml of petroleum ether added, followed by addition of 5 ml distilled water. The solution was evaporated to dryness on the water bath and 6 ml of Liebermann Burchard reagent was added to the residue in dry bottle and absorbance taken at 620 nm on a Spectronic21D digital Spectrophotometer. Standard steroid solutions of 0–4 mg/ml concentration were prepared and treated like sample14,15,16. The percentage steroid was calculated using the formula:
Phlobatannin Determination
The method of Analytical methods committee of the Royal Society of Chemistry14 was employed. About 0.5 g of sample was weighed into 50 ml beaker, 20 ml of 50% methanol was added and covered with parafilm and placed in a water bath set at 77 – 800C for 1 hr. The mixture was properly shaken and then filtered through a Whatman No 1 Filter paper into a 50ml volumetric flask using aqueous methanol to rinse and make up to mark with distilled water. To 1 ml of the solution pipetted into a 50 ml volumetric flask, were added 20 ml water, 2.5 ml Folin-Dennis reagent, and 10 ml of 17% sodium carbonate. This mixture was homogenized thoroughly for 20 mins. Standard Phlobatannin solutions of 0-5 mg/ml concentrations were prepared and treated as the sample above. The absorbance of standard solutions and the sample was read at 550 nm on a Spectronic21D Spectrophotometer. Percent Phlobatannin was calculated using the formula:
Anthraquinones Determination
About 0.50 g of sample was weighed into 250 ml beaker and 60 ml benzene added and stirred and filtered using Whatman No.1 filter paper. About 10 ml of filtrate was pipetted into another 100 ml volumetric flask and 0.2% Zinc dust was added followed by the addition of 50 ml hot 5% NaOH solution. The mixture was heated just below boiling point for 5 mins and then rapidly filtered and washed once in water. The filtrate was again heated with another 50 ml of 5% NaOH to develop a red colour. Standard anthraquinone solutions of range 0-5 mg/l were prepared and treated with 0.2% Zinc dust and NaOH as the sample. The absorbance of sample as well as that of standard solutions were read on a digital spectrophotometer at 640 nm14. The percentage anthraquinone was calculated using the formula:
Terpene Determination
About 0.50g of sample was weighed into a 50 ml conical flask, 20 ml of 2:1 chloroform-methanol mixture was added, thoroughly agitated and allowed to stand for 15 mins. The mixture was later centrifuged for another 15 mins. The supernatant solution obtained was discarded and the precipitate re-washed with another 20 ml chloroform-methanol mixture for re-centrifugation. The resultant precipitate was dissolved in 40 ml of 10% sodium dodecyl sulphate solution, 1 ml of 0.01 M ferric chloride solution added at 30 s interval, shaken well and allowed to stand for 30 mins. Standard terpene solutions of concentrations 0 – 5mg/ml were prepared from stock solution (Sigma-Aldrich chemicals U.S.A). The absorbance of sample as well as the standard solutions of terpenes were read on a digital spectrophotometer at 510 nm14. The percentage terpene was calculated using the formula:
Phenol Determination
About 0.20 g of sample was weighed into a 50 ml beaker, 20 ml of acetone added and homogenized for 1hr and filtered through a Whatman No.1 filter paper into a 100 ml volumetric flask using acetone to rinse, and made up to the mark with distilled water. One millilitre of sample extract was pipetted into 50 ml volumetric flask, 20 ml water added, 3 ml of phosphomolybdic acid added, followed by the addition of 5 ml of 23% Na2CO3 mixed thoroughly, made up to mark with distilled water and allowed to stand for 10 mins to develop bluish-green colour. Standard solutions of phenol of 0-10 mg/ml was prepared from stock solution (Sigma-Aldrich chemicals U.S.A). The absorbance of sample and the standard solutions of phenol were read on a digital spectrophotometer at 510 nm14. The percentage Phenol is calculated using the formula:
Anti-nutrients Determination
Phytate Determination
About 2 g of each sample was weighed into 250 ml conical flask, 100 ml of 2% hydrochloric acid was added, soaked for 3 hrs, filtered through a double layer of hardened filter paper and 50 ml of each filtrate placed in 250 ml conical flask and 107 ml distilled water added. About 10 ml of 0.3% ammonium thiocyanate (NH4SCN) solution was added to each solution and titrated with 0.00195g/ml iron (III) chloride solution to a slightly brownish-yellow end point which persisted for 5 mins12. The percentage phytic acid was calculated using the formula:
Saponin Determination
The spectrophotometric method of Brunner17 was used as adopted by Mujeeb et al., 18. One gramme of finely ground sample was weighed into a 250 ml beaker and 100 ml of isobutyl alcohol was added. The mixture was shaken on a UDY shaker for 5 hrs, filtered through a Whatman No1 filter paper into a 100 ml beaker and 20 ml of 40% saturated solution of magnesium carbonate was added and filtered through a Whatman No1 filter paper to obtain a clear colorless solution. One millilitre of the colorless solution was pipetted into 50 ml volumetric flask and 2 ml of 5% FeCl3 solution added and made up to mark with distilled water. It was allowed to stand for 30 mins for blood red colour to develop. Standard saponin solutions of concentration range 0 – 10 ppm were prepared and treated with 2 ml of 5% FeCl3 solution as done for sample above. The absorbance of the sample and standard saponin solutions were read on a Jenway V6300 Spectrophotometer at 380 nm.
Tannin Determination
The total tannin was determined by the Spectrophotometric procedure described by Association of Official Analytical Chemists12 by measuring 0.20 g of sample into a 50 ml beaker followed by addition of 20ml of 50% methanol, covered with parafilm and placed in a water bath set between 77-800C for 1 hr and shaken thoroughly. The extract was quantitatively filtered using a double layered Whatman No 41 filter paper into a 100 ml volumetric flask. Twenty millilitres (20 ml) water was added, 2.5 ml Folin-Denis reagent and 10 ml of 17% Na2CO3 were added and mixed properly. The mixture was made up to the mark with distilled water and allowed to stand for 20 min for the bluish–green colour to develop. Standard solutions of tannic acid were prepared and treated similarly as the samples. The absorbance of the tannic acid standard solutions as well as samples were read after colour development on a spectronic21D spectrophotometer at 760 nm12. Percent tannin was calculated using the formula:
Oxalate Determination
Two grammes (2 g) of sample was boiled in 40 ml of water for 30 mins in a reflux condenser followed by addition of 10 ml of 20% Na2CO3 and boiled for another 30 mins. The solution was filtered and washed with hot water until the wash water did not show any alkaline reaction. The combined wash water and filtrate were concentrated together to a small volume and cool. With constant stirring, HCl (1:1) was added dropwise until the final acid concentration after neutralization was about 4%, at which stage a heavy precipitate appears (which is allowed to flocculate). The extract was carefully filtered into a 250 ml flask, made up to mark and kept overnight. The supernatant liquid was filtered through a dry filter paper in a dry beaker. An aliquot of the filtrate was taken into a 400 ml beaker, diluted with water to 200 ml and made just ammoniacal, and then reacidified with lactic acid, and 10 ml of the resultant solution added to cold medium of a 10% calcium chloride solution and stirred well to allow calcium oxalate precipitate to appear, and then allowed to settle overnight. The clean supernatant liquid was carefully decanted off through Whatman No. 42 filter paper, without disturbing the precipitate. The precipitate was dissolved in HCl (1:1) and oxalic acid was re-precipitated by adjusting the pH with ammonium hydroxide solution. Content was allowed to boil and settled overnight, while oxalic acid was determined by titrating against 0.05N KMnO4 solution19. Oxalate content was estimated as:
1ml of 0.05N KMNO4=0.00225 M anhydrous Oxalic Acid
= % Oxalic Acid
Chemical analyses were done in triplicate, and data were presented using means and standard deviation.
Results
Proximate Composition of the Spices
The results of the proximate composition of the spices studied are presented in Table 1. The percentage moisture content varied from 11.74 g in Thymus vulgaris to 59.36 g in Ocimumgratissimum. Ocimumgratissimum, Capsicum chinense Linn, Allium sativum and Zingiberofficinale were high in moisture content, constituting about half of the spices on fresh weight basis, while it constituted almost one-third of the constituents of Curcuma longa Linn, Syzgiumaromaticum, and Myristicafragrans. Thymus vulgaris had the lowest value, being usually used in dry form.
Table 1. Proximate composition of selected Spices (g/100g, fresh weight basis ‘As consumed’)| Sample | Moisture | Crude protein | Crude fat | Crude fibre | Ash | Carbohydrate |
| 1 | 58.3±0.04 | 5.5±0.02 | 2.5±0.03 | 3.3±0.02 | 0.9±0.03 | 29.5±0.02 |
| 2 | 28.8±0.03 | 15.1±0.08 | 3.0±0.02 | 11.2±0.02 | 1.9±0.03 | 40.0±0.05 |
| 3 | 38.2±0.03 | 9.3±0.03 | 2.5±0.02 | 8.3±0.02 | 1.2±0.02 | 40.5±0.03 |
| 4 | 48.9±0.03 | 5.6±0.02 | 2.1±0.02 | 4.1±0.02 | 1.3±0.02 | 38.0±0.04 |
| 5 | 35.2±0.04 | 5.9±0.01 | 8.3±0.02 | 2.0±0.02 | 1.7±0.01 | 46.9±0.03 |
| 6 | 11.7±0.03 | 14.9±0.04 | 3.4±0.02 | 11.4±0.05 | 1.8±0.03 | 56.8±0.07 |
| 7 | 59.4±0.02 | 10.1±0.04 | 1.7±0.02 | 6.8±0.02 | 1.7±0.03 | 20.3±0.05 |
| 8 | 55.3±0.02 | 3.7±0.02 | 1.3±0.01 | 2.1±0.02 | 0.6±0.02 | 37.0±0.03 |
The crude protein content of the spices ranged from 3.72 g in Allium sativum to 15.1 g in Myristicafragrans. Allium sativum, Capsicum chinense Linn, Zingiberofficinale and Curcuma longa Linn were low, while Syzgiumaromaticum, Ocimumgratissimum, Thymus vulgaris and Myristicafragrans were relatively high in crude protein. All the spices were very low in crude fat with Allium sativum having the lowest value and Curcuma longa Linn having the highest value. Curcuma longa Linn was lowest in fibre content while Thymus vulgaris had the highest value. Thymus vulgaris and Curcuma longa Linn were high in fibre content, Syzgiumaromaticum, Ocimumgratissimum and Zingiberofficinale contained appreciable amount, and Capsicum chinense Linn, Allium sativum and Curcuma longa Linn were relatively low in fibre content.
The spices contained substantial amount of ash, Myristicafragrans having highest value, followed by Thymus vulgaris, Curcuma longa Linn and Ocimumgratissimum. Allium sativum had the lowest ash value. Carbohydrates constituted not less than one-fifth of the constituents of the spices. Ocimumgratissimum had the lowest carbohydrate content, followed by Capsicum chinense Linn, Allium sativum and Zingiberofficinale, while Thymus vulgaris had the highest value of carbohydrates. Ocimumgratissimum was highest in moisture, Myristicafragrans was highest in protein and ash values, Curcuma longa Linn was highest in fat content, while Thymus vulgaris was highest in fibre and carbohydrate content.
Mineral Composition of the Spices
The mineral content of the selected spices is presented in Table 2. All the spices were high in sodium, potassium, calcium, magnesium, phosphorus and iron content, except Allium sativum, which was low in sodium, calcium, magnesium, and phosphorus content. Thymus vulgaris was highest in sodium, calcium, magnesium, iron, zinc, and copper content; while Myristicafragranswas highest in potassium, phosphorus, manganese and selenium content. Allium sativum had the lowest value for all the minerals studied.
Table 2. Mineral composition of selected Spices (mg/100g fresh weight basis ‘As consumed’)| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Sodium | 114.5±0.20 | 259.9±0.20 | 206.8±0.20 | 169.0±0.16 | 95.41±0.20 | 317.0±0.20 | 146.4±0.30 | 60.6±0.10 |
| Potassium | 408.9±0.50 | 739.6±0.30 | 361.5±0.16 | 553.2±0.30 | 505.2±0.30 | 342.1±0.20 | 845.0±0.12 | 176.3±0.20 |
| Calcium | 111.3±0.20 | 223.0±0.20 | 178.8±0.20 | 153.4±0.40 | 125.0±0.30 | 423.9±0.30 | 223.6±0.28 | 78.5±0.20 |
| Magnesium | 124.6±0.24 | 260.7±0.20 | 201.2±0.14 | 176.4±0.16 | 136.4±0.28 | 322.1±0.30 | 143.6±0.28 | 82.0±0.20 |
| Phosphorus | 172.1±0.20 | 340.1±0.20 | 272.7±0.20 | 230.5±0.20 | 235.0±0.20 | 279.5±0.30 | 223.6±0.28 | 78.5±0.20 |
| Iron | 8.49±0.20 | 15.68±0.16 | 13.45±0.34 | 10.82±0.02 | 9.11±0.25 | 20.10±0.21 | 8.99±0.04 | 5.78±0.04 |
| Zinc | 1.71±0.25 | 3.46±0.25 | 2.79±0.29 | 2.27±0.01 | 2.26±0.06 | 4.31±0.21 | 2.01±0.03 | 1.41±0.20 |
| Copper | 0.29±0.01 | 0.60±0.02 | 0.41±0.03 | 0.30±0.03 | 0.36±0.02 | 0.68±0.02 | 0.35±0.02 | 0.17±0.02 |
| Manganese | 0.34±0.02 | 0.92±0.05 | 0.66±0.02 | 0.49±0.02 | 0.56±0.03 | 1.05±0.03 | 0.52±0.02 | 0.32±0.02 |
| Selenium (µg/) | 2.17±0.02 | 4.97±0.03 | 3.01±0.02 | 2.87±4.24 | 1.64±0.03 | 5.09±0.02 | 2.65±0.03 | 1.99±0.01 |
Quantitative Phytochemical Analysis
The phytochemical contents of the extract of the studied spices are presented in Table 3. Flavonoids was found to be the most abundant phytochemical in Cameroon pepper (31.673%) but was present in very minute concentration in the remaining spices. The concentrations of total phenol ranged between 0.003 and 0.276%, glycosides 0.075 and 0.116%, and phlobatanin 0.006 and 0.034%. Steroid content ranged between 0.002% in garlic and 0.041% in clove. Ginger had the highest value of anthraquinones while garlic had the lowest concentration. The content of terpenes in the analyzed samples ranged between 0.075 - 0.116%, with Ocimumgratissimum having the highest value while Allium sativum had the lowest value.In Table 4, all the analyzed spice samples were low in antinutrient content. Ginger had the highest concentration of phytate (0.374%) while its least concentration of 0.059% was found in turmeric.
Table 3. Phytochemical composition of selected Spices (% Dry weight basis)| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Flavonoids | 31.673±4.78 | 0.008±0.002 | 0.007±0.000 | 0.006±0.000 | 0.005±0.000 | 0.007±0.000 | 0.009±0.002 | 0.003±0.001 |
| Total phenol | 0.265±0.002 | 0.276±0.000 | 0.251±0.003 | 0.003±0.000 | 0.271±0.002 | 0.261±0.002 | 0.174±0.002 | 0.149±0.002 |
| Glycosides | 0.105±0.001 | 0.116±0.002 | 0.090±0.002 | 0.094±0.002 | 0.091±0.002 | 0.105±0.002 | 0.114±0.002 | 0.075±0.002 |
| Phlobatanin | 0.023±0.002 | 0.034±0.001 | 0.015±0.002 | 0.013±0.002 | 0.016±0.001 | 0.021±0.002 | 0.026±0.001 | 0.006±0.000 |
| Steroid | 0.028±0.003 | 0.005±0.000 | 0.041±0.001 | 0.008±0.000 | 0.004±0.000 | 0.004±0.000 | 0.005±0.000 | 0.002±0.000 |
| Anthraquinones | 0.008±0.000 | 0.009±0.000 | 0.006±0.002 | 0.168±0.002 | 0.008±0.000 | 0.008±0.002 | 0.009±0.000 | 0.005±0.000 |
| Terpenes | 0.005±0.000 | 0.005±0.000 | 0.003±0.000 | 0.003±0.000 | 0.004±0.002 | 0.005±0.000 | 0.006±0.000 | 0.001±0.000 |
| Parameter | Phytate | Oxalate | Tannin | Saponin |
| 1 | 0.094±0.002 | 0.056±0.002 | 0.051±0.002 | 0.278±0.002 |
| 2 | 0.119±0.002 | 0.095±0.002 | 0.252±0.260 | 0.297±0.004 |
| 3 | 0.094±0.002 | 0.074±0.002 | 0.046±0.002 | 0.268±0.003 |
| 4 | 0.374±0.392 | 0.057±0.003 | 0.029±0.002 | 0.155±0.002 |
| 5 | 0.059±0.002 | 0.026±0.001 | 0.028±0.003 | 0.110±0.002 |
| 6 | 0.105±0.002 | 0.088±0.002 | 0.054±0.002 | 0.279±0.245 |
| 7 | 0.116±0.002 | 0.097±0.001 | 0.061±0.003 | 0.296±0.002 |
| 8 | 0.068±0.001 | 0.032±0.002 | 0.015±0.001 | 0.118±0.001 |
Discussion
In Table 1, the spices were high in moisture content except Thymus vulgaris. The high moisture content of the spices provides for greater activity of water-soluble enzymes and co-enzymes needed for metabolic activity20. The moisture values reported here for Zingiberofficinale and Ocimumgratissimum are significantly lower than the value reported in the literature21,22, and are different from values reported by other researchers23. The observed differences in values could be as a result of seasonal and geographic variations; as these samples were obtained from different geographic locations and years.
The amount of crude protein in the Ocimumgratissimum leaf was at variance with those reported in the literature22,23. It has been reported that plant foods that provide more than 12% of their caloric value from protein are of good sources of protein24, hence, it can be inferred that two of the eight spices investigated (i.e. Thymus vulgaris and Myristicafragrans)had their values above 12% (12 g /100 g protein) which make them to be termed as good sources of protein. However, the other spices can contribute to protein intake of their consumers if the protein content in them is bio-available. The lowest concentration of crude fat was recorded in Allium sativum while Curcuma longa had the highest concentration. These values were fairly similar when compared with values reported in the literature23, but the spices may not be good sources of fat-soluble vitamins.
The crude fibre content of the spices fell within the range from the reported values of some Nigerian spices and vegetables25. Dietary fibre helps to prevent constipation, aid bowel health and weight management26, hence, consumption of these spices can help in promoting good health and wellness. The spices were relatively high in ash content, indicating that they will be rich in mineral content, especially the macrominerals. The values obtained for the spices in this study are comparable to those reported in the literature20,27. The spices can contribute to carbohydrate intake of consumers.
Mineral Content of the Spices
Potassium was the most abundant mineral in all the spices. This finding is in agreement with the report by Shaaban and Moawad28, that reported potassium as the main macro essential element in the tested spices and herbs analyzed while on the other hand, copper was the least abundant trace element within all the samples. The highest value of sodium was obtained in Thymus vulgaris, while Allium sativum had the lowest sodium content. The value of sodium reported in this study for Zingiberofficinale was lower than that of Bamigboye et al.,29. In comparison to other major elements evaluated in this study, sodium was found in low amounts. The presence of sodium in any food item is responsible for the regulation of plasma volume, acid-base balance, nerve and muscle contraction30 with potassium and sodium as important intracellular and extracellular cations respectively.
Potassium was the most abundant element in all spices, the highest concentration being found in Myristicafragrans. However, the potassium range of values were significantly lower compared with that of medicinal and aromatic plants used as spices, condiments and herbal teas (357 – 2767mg/100g)31. The second abundant element found in all the samples was phosphorus, with the highest value in Myristicafragrans. Phosphorus is essential in the body for filtering of waste, repairing of tissue and cells, and the deficiency of phosphorus – calcium balance results in osteoporosis, arthritis, pyorrhea, rickets and tooth decay27.
All the spices except Allium sativum were high in calcium content. The concentrations of calcium in Allium sativum and Curcuma longa samples were comparable with the results obtained from Canary Island32 and Ethiopia red pepper33 but higher than the values reported by some studies34,35. Magnesium content was lowest in Allium sativum and highest in Thymus vulgaris. The results of this study were higher than those reported by Asaolu et al.,27 and much lower than that of Tchiegang and Mbougueng36. All the spices contained substantial amount of iron, with the highest value observed in Thymus vulgaris and lowest value in Allium sativum. The values of iron obtained in this study were higher than those reported by Bamigboye et al.,29, and Akpanyung30, but lower than other values reported in the literature35,38,39,40. Iron is an important trace element in the human body that plays crucial roles in hemopoiesis, control of infection and cell mediated immunity27. Deficiency of iron has been described as the most prevalent nutritional deficiency, and iron deficiency anemia is estimated to affect more than one billion people worldwide41. The consequences of iron deficiency include reduced work capacity, impairments in behavior and intellectual performance and decrease resistance to infection42.
The concentration of zinc in all the samples studied are lower than those reported for spices in Federal Capital Territory, Abuja, Nigeria43,44. Zinc is an essential element that enhances growth, and also participate in some enzymes structure or their catalytic and regulatory action45. According to World Health Organisation (WHO), permissible limits of zinc is 100mg/kg for spice43, but the concentrations of zinc (mg/kg) for all the spices studied were well below the permissible limit of WHO, and hence, may be considered tolerable.
All the values obtained for copper in this study were below the regulatory WHO/FAO limit (50mg/kg) for copper in spices and seasonings, hence, its concentrations in all the samples do not pose health risks. Manganese concentration in all the analyzed samples was below the WHO recommended limit, which can be considered tolerable for all the samples. Zingiberofficinale was high in selenium. Generally, the low level of Se in foods has been ascribed to low selenium in soil content47. It has been demonstrated that some plants known as Selenium-accumulator, highly absorb selenium compared to non selenium-accumulator plants. The high selenium content offers dietary selenium sources for the management of several chronic diseases such as cardiovascular diseases, cancers and HIV48.
The values of flavonoids obtained in this study were significantly different from those in the literature49,50. Flavonoids have antioxidant activities, antimicrobial properties as well as much health promoting effects such as anti-allergic, antispasmodic, anti-cancer, antidiabetic, hypoglycaemic, anti-inflammatory, anti-thrombotic, vaso-protective, tumour inhibitory and anti-viral effects51,52. The values of phenolic content of the spices were lower than those in the literature. Phenolic compounds possess both antioxidant and antimicrobial activities53,54,55, while glycosides are useful in lowering blood pressure, treatment of congestive heart failure and cardiac arrhythmia56. Steroidal compounds are of importance and interest in pharmacy due to their relationship with compounds such as sex hormones mostly used in the development of female contraceptive pills54. This can make these spices useful for pregnant women and breastfeeding mothers to ensure their hormonal balance, as being used in some countries since steroidal compounds could serve as essential starting material in the synthesis of these hormones50. Anthraquinones possess laxatives, anti-malaria and anti-carcinogenic effects47. The presence of terpenes in food items has been reported to be useful in the treatment of cough, asthma and hay fever56.
All the spices were low in antinutritional factors. The anti-nutritional activity of phytates can also be beneficial especially in menopause women and most adults, as they tend to have high levels of iron which can be a very strong oxidant and causes biological stress50. Oxalates possess certain health benefits especially when present at low concentration by maintaining the levels of certain minerals in the body. Tannin acts as an antioxidant also has biological property like anti-carcinogen, anti-inflammation, cardiovascular protection and cell proliferation activities57. Saponins have potential of inhibiting tumor in animals and also used for traditional medicine preparation57. The presence of phytochemicals coupled with low levels of antinutrients which doubles as phytochemical may be the possible reasons why the spices possess health-promoting properties.
Conclusion
The selected spices contained appreciable amounts of crude protein, fibre and carbohydrates. Although, they are not major sources of nutrients but used mainly to add flavor, aroma and taste to food and dishes, they can contribute meaningfully to meeting the recommended dietary allowances of people, especially the essential minerals. The microminerals/heavy metals are present at permissible level of heavy metals as stipulated by World Health Organisation, indicating that their usage in food preparation and consumption on daily basis is safe with no risk of toxicity. The presence of natural antioxidants and phytochemicals in the spices can help in building up immunity and prevention of non-communicable diseases, hence, their inclusion in foods should be encouraged. The study has revealed that these spices have the potential of contributing to the nutritional and health needs of human beings.
Consent and Ethical Approval
It is not applicable.
References
- 1.Onyema C T, Ekpunobi U E, Ndigbo E O. (2015) Evaluation of the heavy metals level in selected industrially packaged food spices.American Association for Science and Technology. 2(2), 35-40.
- 2.Deepali D K, Pramod C M, Ravindra D C. (2013) Phytochemical screening and pharmacological applications of some selected Indian spices. , International Journal of Science and Research 3, 704-706.
- 3.Ene-Obong H N, Onuoha N O, Aburime L C, Mbah O.Nutrient composition, phytochemicals and antioxidant activities of some indigenous spices in Southern Nigeria. 11, 1-31.
- 4.Valko M, Leibfritz D, Moncol J, Cronin M T, Mazur M et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. , The International Journal of Biochemistry and Cell Biology 39, 44-84.
- 5.Hinneburg I, DHJ Damien, Hiltunen R. (2006) Antioxidant activities of extracts from selected herbs and spices.Food Chemistry. 97(1), 122-129.
- 6.Lampe J W. (2003) Spicing up a vegetarian diet: chemopreventive effects of phytochemicals.American. , Journal of Clinical Nutrition 78(3), 579-583.
- 7.Gupta C, Garg A P, Uniyal R C. (2009) Antimicrobial and Phytochemical studies of Amchur (Dried Pulp of UnripeMangiferaindica) exract on some food borne bacteria. , The Internet Journal of Tropical Medicine 5(2), 1540-2681.
- 8.Santhi K, Sengottuvel R. (2016) Qualitative and Quantitative phytochemical analysis ofMoringaconcanensisNimmo. , International Journal of Current Microbiology and Applied Sciences 5(1), 633-640.
- 9.Kabelitz L, Sievers H, Kaur J. (2004) Antimicrobial activity of spices. , Journal of Antimicrobial Agents 12, 257-259.
- 10.Divrikli U, Horzum N, Soylak M, Elci L. (2006) Trace heavy metal contents of some spices and herbal plants from western Anatolia. , Turkey, International Journal of Food Science & Technology 41(6), 712-716.
- 11.Jordan S A, Cunningham D G, Marles R J. (2010) Assessment of herbal medicinal products: challenges and opportunities to increase the knowledge base for safety assessment. Toxicology and Applied Pharmacology. 243(2), 198-216.
- 12.AOAC. (2005) Official Methods of Analysis of the Association of Analytical Chemists. , Washington, D.C., USA
- 13.Radojković M M, Zeković Z P, Vidović S S, Kočar D D, Mašković P Z. (2012) Free radical scavenging activity and total phenolic and flavonoid contents of mulberry (Morusspp. L.,Moraceae) extracts. , Hem. Ind 66(4), 547-552.
- 14.AMC-RSC. (1986) Methods of Analysis of Analytical Methods. , Committee of Royal Society of Chemistry 222-239.
- 15.Wall M E, Eddy C R, McClenna M L, Klump M E. (1952) Detection and estimation of Steroid and Sapogenins in plant tissue. , Analytical Chemistry 24, 1337-1342.
- 16.Maguire L S, O’sullivan S M, Galvin K, O’connor T P, O’brien N M. (2004) Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almond, peanuts, hazelnuts and the macadamia nut. , International Journal of Food Sciences and Nutrition 55(3), 171-178.
- 18.Mujeeb F, Bajpai P, Pathak N. (2014) Phytochemical evaluation, antimicrobial activity and determination of Bioactive components from leaves ofAnglemarmelos. , BioMed Research International 1-11.
- 19.Onwuka G I. (2005) Food analysis and instrumentation. Theory and Practice. Napthali Prints. 140-146.
- 20.Iheanacho K.Ubebani AC (2009). Nutritional composition of some leafy vegetable consumed in Imo State. , Nigeria. J. Appl. Sci. Environ. Manage 13(3), 35-38.
- 21.Omotayo O A, Adepoju O T.Keshinro OO (2013). Evaluation of micronutrient potentials of seven commonly consumed indigenous spices from Nigeria. , American J. Food Nutri 3(3), 122-126.
- 22.Adepoju O T, Adediji R A, Adigwe P C. (2012) Nutrient composition and micronutrient potentials of fresh and processed tree basil (Ocimumgratissimuml)leaf. , African Journal of Medicine and Medical Sciences 41(1), 75-80.
- 23.Nwinuka N M, Ibeh G O, Ekeke G I. (2005) Proximate composition and levels of some toxicants in four commonly consumed spices. , J. Appl. Scvi. Environ. Mgt 9(1), 150-155.
- 24.Ali A. (2009) Proximate and mineral composition of the marchbeh (Asparagus officinalis). World Dairy and Food Science. 4(2), 142-149.
- 25.Uusiku N P, Oelofse A, Duodu K G, Bester M J, Faber M. (2010) Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: A review. Journal of food composition and analysis. 23(6), 199-509.
- 26.Rolfes S R, Pinna K, Whitney E. (2009) Understanding normal and clinical nutrition. , Eighth Edn Wadsworth Cengage Learning 122-123.
- 27.Asaolu S S, Adefemi O S, Oyakilome I G, Ajibulu K E, Asaolu M F. (2012) Proximate and mineral composition of Nigerian Leafy Vegetables. , Journal of Food Research 1(3), 214-218.
- 28.Shaaban H A, Moawad S A. (2017) Chemical composition, nutritional and functional properties of some herbs and spices. Current Science Perspectives. 3(4), 165-179.
- 29.Bamigboye A Y, Adepoju O T, Oyinsan O, Kabir H. (2012) Micronutrient potentials and contribution to nutrient intake of four commonly consumed local condiments and spices in South-Western Nigeria. , Nigerian Journal of Nutritional Sciences 33(1), 57-61.
- 30.Akpanyung E O. (2005) Proximate and mineral composition of bouillon cubes produced in Nigeria. , Pakistan Journal of Nutrition 4(5), 327-329.
- 31.Ezeagu I E, Ibeagu M D. (2010) Biochemical and Nutritional potential of Ukpa: A variety of Tropical Lima Beans (Phaseoluslunatus) from Nigeria – A short Report. , Poland Journal of Food Nutrition Science 60(3), 231-235.
- 32.Rubio C, Hardisson A, Martin R E, Baez A, Martin M M et al. (2002) Mineral composition of the red and green pepper (Capsicum annuum) from Tenerife Island. European Food and Research Technology. 214, 501-504.
- 33.Tefera M, Chandravanshi B S. (2018) Assessment of metal contents in commercially available Ethiopian red pepper. , International Food Research Journal 25(3), 989-1000.
- 34.Ismail F, Anjum M R, Mamon A N, Kazi T G. (2011) Trace metal contents of vegetables and fruits of Hyderabad retail market. , Pakistan Journal of Nutrition 10, 365-372.
- 35.Ekholm P, Reinivuo P, Mattila H, Pakkala H, Koponen J et al. (2007) Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. Journal of Food composition and Analysis. 20(6), 487-495.
- 36.Tchiegang C, Mbougueng D. (2005) Composition Chimique des Epices Utilisées dans la Préparation du na’a poh et du kui de l’Ouest Cameroun. , Tropicultura 23(4), 193-200.
- 37.Soylak M, Tuzen M, Narin I, Sari H. (2004) Comparison of microwave, dry and wet digestion procedures for the determination of trace metal contents in spice samples produced in Turkey. , Journal of Food and Drug Analysis 12, 254-258.
- 38.Bidgeli M, Seilsepour M. (2008) Investigation of metals accumulation in some vegetables irrigated with waste water in Shahre Rey-Iran and toxicological implications. , American-Eurasian Journal of Agriculture and Environmental Science 4, 86-92.
- 39.Trowbridge F, Martorell M. (2002) Forging effective strategies to combat iron deficiency. Summary and Recommendations. , J. Nutri 85, 875-880.
- 40.Dixon B M, Akinyele I O, Oguntona E B, Nokoe S, Sanusi R A et al. (2004) Nigeria Food Consumption and Nutrition Survey. Summary IITA. , Ibadan. Nigeria
- 41.Umar M A, OOS Zubair. (2014) Heavy metals content of some spices available within FCT. , Abuja, Nigeria, International Journal of Agricultural and Food Science 4(1), 66-74.
- 42.Onianwa P C, Adeyemo A O, Idowu A O, Ogabiela E E. (2001) Copper and zinc contents of Nigerian foods and estimates of the adult dietary intakes. Food Chemistry. 72, 89-95.
- 43.Nkansah M A, Amoako O. (2010) Heavy metal content of some common spices available in markets in Kumasi metropolis of Ghana. , America Journal of Scientific and Industrial Research 1(2), 158-163.
- 44.World Health Organization. (2005) Quality control methods for medicinal plant materials. , Geneva
- 45.Bouba A A, Njintang N Y, Foyet H S, Scher J, Montet D et al. (2012) Proximate composition, mineral and vitamin content of some wild plants used as Spices in Cameroun. Food and Nutrition Sciences. 3, 423-432.
- 46.Sirichakwal P, Puwastien P, Polngam J, Kongkachuichai R. (2005) Selenium content of Thai foods. , Journal of Food Composition and Analysis 18(1), 47-59.
- 47.Oloyede O B, Oladiji A T.Afolayan AJ (2010). Comparative analysis of the chemical composition of three spices –Alliunsativum L,ZingiberofficinaleRoscandCapsicumfrutescensL.commonly consumed in Nigeria. , African Journal of Biotechnology 41, 6927-6931.
- 48.Akeem S, Joseph J, Kayode R, Kolawole F. (2016) Comparative phytochemical analysis and use of some Nigerian spices. , Croatian Journal of Food Technology, Biotechnology and Nutrition.11(3-4): 145-151.
- 50.Tanko Y, Yaro H A, Isa M, Yerima M, Saleh I A et al. (2007) Toxicological and hypoglycaemic studies on the leaves ofCissampelosmucronata(Menispermaceae) on blood glucose levels of streptozotocin-induced diabetic wistar rats. , Journal of Medicinal Plants Research 1(5), 113-116.
- 51.Zheng W, Wang S Y. (2001) Antioxidant activity and phenolic compounds in selected herbs. , Journal of Agricultural and Food Chemistry 49(11), 5165-5170.
- 52.Virgili F, Scaccini C, Packer L, Rimbach G. (2001) Cardiovascular disease and nutritional phenolics. In: Pokorny. , Cambridge. Pp 87-99.
- 53.Akharaiyi F C, Boboye B. (2010) Antibacterial and Phytochemical Evaluation of Three Medicinal Plants. , Journal of Natural Products 3, 27-34.
- 54.Kadam D D, Mane P C, Chaudhari R D. (2015) Phytochemical screening and Pharmacological Applications of some selected Indian Spices. , International Journal of Science and Research 4(3), 704-706.
- 55.Okwu D E. (2001) Evaluation of the chemical composition of Indigenous spices and flavoring agents. , Global Journal of Pure and Applied 7(3), 455-459.
Cited by (2)
- 1.Adugna Teshome, Selale Girma, Regassa Girma, 2023, Assessment of Heavy Metal Contents in Some Common Spices Available in the Local Market of North Shewa Zone, Oromia Regional State, Ethiopia, Biological Trace Element Research, (), 10.1007/s12011-023-03921-8
- 2.Okiki Pius A., Nwobi Chizobam P., Akpor Oghenerobor B., Adewole Ezekiel, Agbana Richard D., 2023, Assessment of nutritional and medicinal properties of nutmeg, Scientific African, 19(), e01548, 10.1016/j.sciaf.2023.e01548