Abstract
Background:
Oral ingestion of zinc oxide nanoparticles (ZnONPs) may lead to serious liver injury. Vitamin E (VE) is an important antioxidant factor that can reduce such damage.
Aim:
This study aimed to evaluate the possible changes that could take place in the liver of adult male albino rats after oral ingestion of ZnONPs and elucidate the potential protective role of VE against such damage.
Material and Methods:
Forty eight male albino rats were divided into four groups of 12 animals each. Group (1) served as control group and received normal saline. Group (2) “VE-treated” received 100 mg/kg/day of VE dissolved in normal saline by oral gavage for 21 days. Group (3) “ZnONPs-treated” received a daily dose of ZnONPs dispersed in the fresh sterilized physiological saline solution 1mg/kg for 5 constitutive days. Group (4) “concomitant ZnONPs and VE-treated” was pretreated with VE 100 mg/kg/day for 14 days followed by the same dose of ZnONPs as in group (3) for 5 days. The extent of hepatic damage was evaluated by histological and immunohistochemical examination of liver samples and serological analysis of liver enzymes.
Results:
Body weights and liver weights showed very highly significant decrease (P <0.001) in the ZnONPs-treated group. The histological results in ZnONPs-treated group revealed congested dilated central veins and blood sinusoids, loss of normal arrangement of hepatocytes and most of hepatocytes showed marked vacuolated cytoplasm with darkly stained nuclei. Portal area affection was in the form of congested dilated portal veins with bile duct hyperplasia and cellular infiltration. There was an increase in the mount of blue stained collagen fibers around central veins together with strong positive reaction for Caspase 3 in ZnONPs-treated group. Similarly biochemical analysis indicated that the levels of serum aminotransferase (AST &ALT) significantly increased in ZnONPs-treated group when compared with other groups. Rats pretreated with VE showed improvement of the histological findings and biochemical parameters.
Conclusion:
Ingestion of ZnONPs could be associated with serious liver affection and pretreatment with VE is suggested to induce some improvement of such deleterious changes.
Author Contributions
Academic Editor: Yuksel Aydar, Department of Anatomy, Medical School of Eskisehir Osmangazi University, Eskisehir, Turkey.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Abdelmonem A. Hegazy, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The liver plays the major role in detoxification process; hepatocytes are considered as a massive detoxification center for degrading alcohol, toxins and drugs protecting the body from their deleterious effects 1. Therefore liver cellular damage commonly resulted from the metabolites of such toxins.
Nanotechnology is a fast developing field that has been used in a wide range of applications including; engineering, agriculture and medicine 2. Nanoparticles are used in some drugs to maximize their bioavailability such as sunscreens, toothpastes, cosmetics, food additives, furniture varnishes and in optic industry e.g. the anti-reflective ultra-thin sunglasses, as well as construction materials 3. ZnONPs are commonly used in food stuffing due to their antifungal properties. In addition, ZnONPs are added to fungicides to increase their effectiveness. They are commonly applied as antibacterial agents in lotions, ointments, and mouthwashes to prevent micro-organisms growth by their bacteriostatic properties 4.
ZnONPs have different physicochemical properties compared to zinc (ZnO) which can affect bioactivity and toxicity of ZnONPs 5. It is suggested that the size of nanoparticles and its surface area greatly increase their ability to produce reactive oxygen species (ROS) 6.
Reactive oxygen species (ROS) that include free radicals are frequently created in cells in normal circumstances as a result of aerobic metabolism. Once cells are exposed to a physical or chemical insult, it causes overproduction of ROS 7. ROS play an important role in nanomaterial induced cytotoxicity. Exposure to NPs leads to occurrence of imbalance between ROS production and antioxidant defense system 8.
The nanoparticles enter the blood circulation in an ionic form and localize in organs as zinc ions. Liver is among the possible target organs for accumulation of zinc ions. They spread to various organs such as kidney and lungs within 72 hours regardless of particle size or charge 9. Toxicity of ZnONPs depends on not only the dose, but also the physicochemical properties of these nanoparticles such as: shape, size, chemical composition, their solubility and route of exposure 10. The gastrointestinal tract is one of the important NPs, oral exposure of nanomaterial can occur directly from water, food, or orally administered medicines 11.
Zinc plays an important role in controlling apoptosis; however excess of zinc may cause cell death due to apoptosis or necrosis. This process is triggered, when the concentrations of intracellular zinc increase 12. Administration of ZnONPs has been suggested to cause apoptosis which considers a key event after oxidative DNA damage. There was evident by marked increase in the activity of the apoptotic biomarker (Caspase 3) in liver tissue 13.
Acute exposure to ZnONPs shows hepatic damage and liver dysfunction demonstrated by increasing alanine transaminase (ALT) and aspartate transaminase (AST) which is located primarily in the cytosol of hepatocytes 14. These results reveal that ZnONPs interrupt the functions of liver cells. Hepatotoxic symptoms included fever, suppression of bile flow in addition to enlargement of liver are common sequences 15.
Vitamin E (VE) is considered to be one of the most potent radical scavengers and a chain breaking antioxidant that interrupts the chain propagation of lipid oxidation, thus protecting polyunsaturated fatty acids and low density lipoproteins from oxidation, hence it stabilize lipid portion of cell membrane and maintains its integrity. For this reason, there is an increasing interest in fortifying many foods with VE 16. In liver, VE (especially α tocophrol) is incorporated into very low density lipoproteins and excreted back into circulation 17. This study aimed to demonstrate the possible adverse effects of ZnONPs on the liver structure and the possible protective role of VE in such case.
Materials and Methods
Animals
The present study was conducted on forty eight adult male albino rats weighting 150-200 gm, with free access to food and water. All experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Faculty of Medicine; Zagazig University.
Chemicals
ZnONPs were purchased from Sigma-Egypt in bottles each bottle was 5 gm., with particle size <50nm. The powdered ZnONPs were dispersed in the fresh sterilized physiological saline solution. Vitamin E was obtained in the form of powder (α-tocopherol) from El Gomhoria Company, Egypt and dissolved in normal saline. Both drugs were given orally via gastric gavage.
Experimental Design
They were equally divided into four groups each contained 12 rats:
1st group (control group):- The animals of this group received physiological saline solution to serve as control group.
2nd group (VE- treated group):- The animals of this group received orally daily dose of VE (100 mg/kg BW) for 21 days 18.
3rd group (ZnONPs-treated group):- The animals of this group received orally daily dose of ZnONPs (1gm/kg/ BW) for 5 constitutive days 19.
4th group (ZnONPs and VE- treated group): The animals of this group pretreated with VE in the same dose of 2nd group for two weeks then combined with ZnONPs (1gm/kg/BW) for another 5 days 20.
Histological and ImmunohistoChemical Study
At the end of the experiment, all rats were weighed, anesthetized and sacrificed by decapitation. The liver of the animals was excised, weighted then fixed in buffered 10% formalin solution for 24 hours. Liver specimens were processed for light microscopic (LM) examination 21. Then they were stained with Hematoxylin and Eosin (H&E) and Masson's trichrome 22 for histological examination using light microscope and Anti-Caspase 3 according to Krajewska et al 23.
Biochemical Assay
Blood samples (2 ml) were collected from the ophthalmic venous plexus through the retro-orbital approach and centrifuged for 10 minutes at 5000 rpm to harvest the clear serum where Liver function tests; (AST) and (ALT) were assessed 24.
Morphometric Study
Sections stained for Masson's trichrome and Caspase 3 was analyzed. Data were obtained using Leica Qwin 500 Image Analyzer Computer System (England).Ten high power field readings were obtained for each group to obtain the mean area percentage of collagen and of caspase-3 expression were calculated automatically by the image analyzer.
Statistical Analysis
The collected data were computerized and statistically analyzed using SPSS program (Statistical Package for Social Science) version 18.0 25. Quantitative data were expressed as mean ± SD (standard deviation). ANOVA F-test test was used to calculate difference between quantitative variables in more than two groups. Least significant difference (LSD) test was used to find significance between each two studied groups. P-value ≤ 0.05 was considered to be significant.
Results
Histological Examination
Examination of H&E stained sections of the 1st group (control group) revealed hepatic architecture of tightly packed cords of hepatocyte with vesicular nuclei and acidophilic cytoplasm radiating from the central vein (Figure 1). Portal area was composed of a portal vein, branch of hepatic artery and bile duct lined by single cuboidal cells with dark rounded nuclei. Blood sinusoids with their endothelial lining of Kuppfer cells were noticed in between hepatic cords (Figure 2). Examination of the liver sections of the 2nd group (vitamin E treated group) showed nearly the same histological features as the 1st group. The 3rd group (ZnONPs-treated group) showed a marked loss of the normal liver arrangement with dilated congested central veins and blood sinusoids. Most of hepatocytes had large cytoplasmic vacuoles and darkly stained nuclei other hepatocytes were ballooned (Figure 3). The portal area showed dilated congested portal vein with proliferation of bile duct, necrotic foci in between the hepatocytes with inflammatory cellular infiltration (Figure 4). The portal area showed also elongation of the endothelial lining of dilated congested portal vein and increasing amount of connective tissue fibers (Figure 5). There was congested hepatic artery with increasing thickness of its muscular layer (Figure 6). Bile duct showed proliferation with stratification of its epithelial lining (Figure 7).
Figure 1.A photomicrograph of a section in the liver of a control adult albino rat showing polygonal hepatocytes (H) radiating from central vein (Cv) with rounded vesicular nuclei and acidophilic cytoplasm. Narrow radiating blood sinusoids (s) in between liver cords and their lining endothelium are noticed. Binucleated cell is also seen (thick arrow). (H&E X400)
Figure 2.A photomicrograph of a section in the liver of a control adult albino rat showing portal area containing bile duct (Bd) and hepatic artery (Ha). Polygonal hepatocytes (H) with rounded vesicular nuclei and acidophilic cytoplasm can be observed. Narrow radiating blood sinusoids (s) in between liver cords and their lining endothelium are seen. Binucleated cells are also seen (thick arrows). (H&E X400)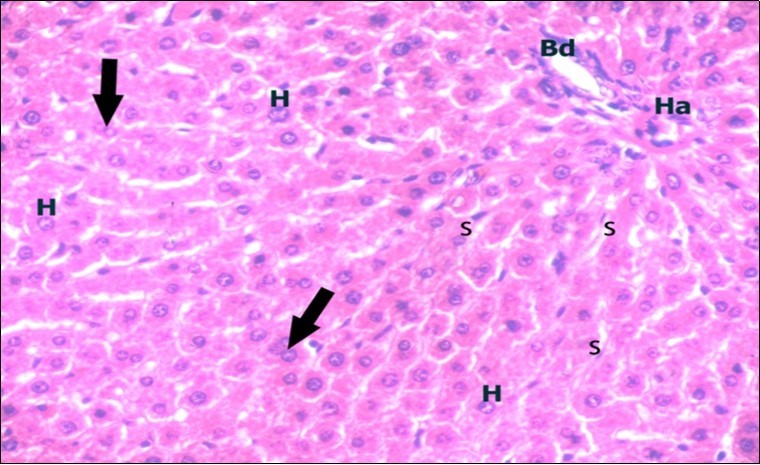
Figure 3.A photomicrograph of a section in the liver of ZnONPs-treated adult albino rat showing dilated congested central vein (Cv) and ballooning of hepatocytes (double arrows) with darkly-stained nuclei (h) and vacuolated cytoplasm (v). Binucleated cells are also seen (thick arrows). (H&E X400)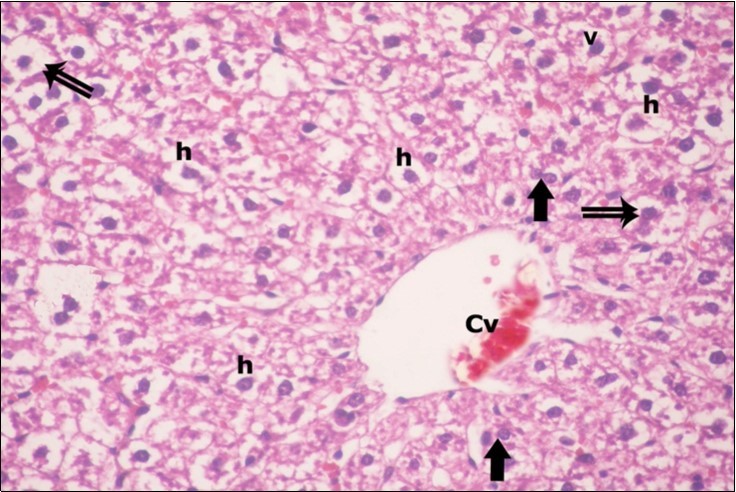
Figure 4.A photomicrograph of a section in the liver of ZnONPs-treated adult albino rat showing dilated congested portal vein (Pv), proliferation of bile duct (Bd), inflammatory cell infiltration (IF) and ballooning of hepatocytes (double arrows) with darkly-stained nuclei (h) and vacuolated cytoplasm (v). Area of necrotic focus is also present (star). (H&E X400)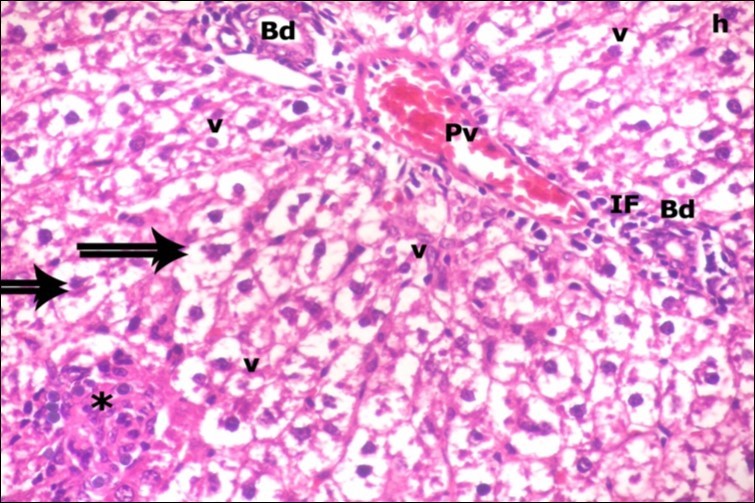
Figure 5.A photomicrograph of a section in the liver of ZnONPs-treated adult albino rat showing congested portal vein (Pv) with elongation of its endothelial lining (arrow head) and increasing amount of connective tissue fibers (Cf). Mononuclear cellular infiltration (IF) and hepatocytes with darkly-stained nuclei (h) and vacuolated cytoplasm (v) could be demonstrated. (H&E X400)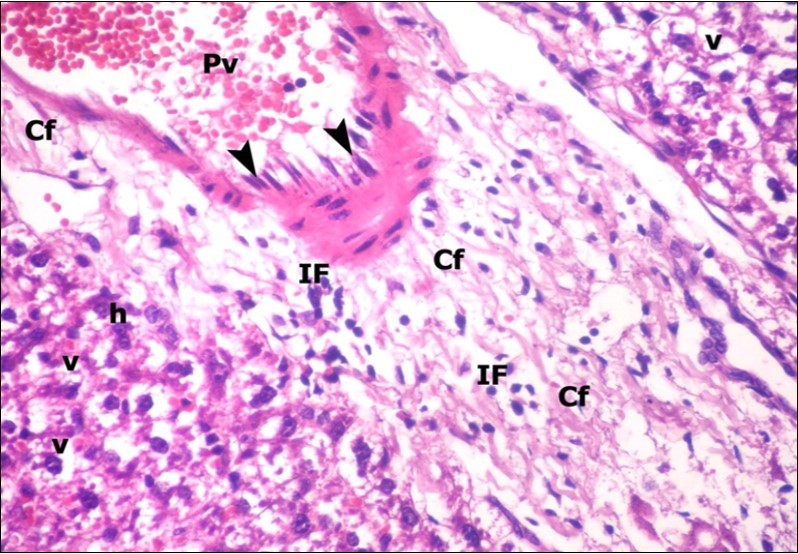
Figure 6.A photomicrograph of a section in the liver of ZnONPs-treated adult albino rat showing congested hepatic artery (Ha) with increasing thickness of its muscular layer (M) and proliferation of bile duct (Bd). Mononuclear cellular infiltration (IF), congested blood sinusoids (s) and hepatocytes with darkly-stained nuclei (h) and vacuolated cytoplasm (v) are also seen. (H&E X400)
Figure 7.A photomicrograph of a section in the liver of ZnONPs-treated adult albino rat showing proliferation of bile duct (Bd) with stratification of its epithelial lining (E). Mononuclear cellular infiltration (IF) in the portal area and hepatocytes with darkly-stained nuclei (h) and vacuolated cytoplasm (v) are also seen. (H&E X400)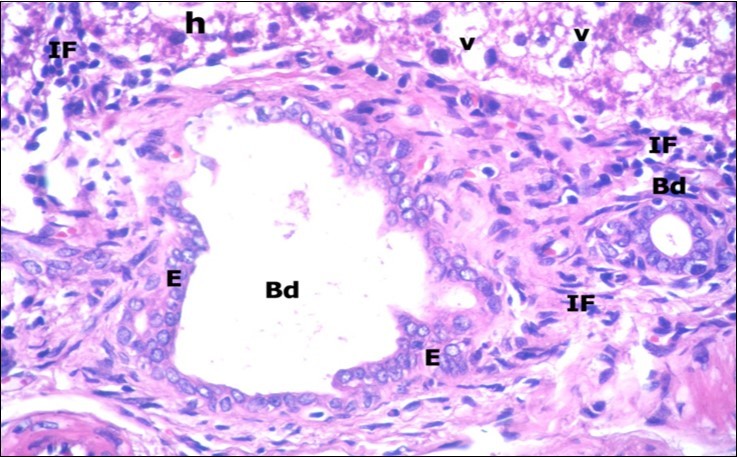
Examination of H&E stained liver sections of the 4th group (ZnONPs &VE-treated group) showed variable degrees of improvement with slightly preserved liver architecture when compared to that of the ZnONPs-treated group. Some hepatocytes showed vacuolated cytoplasm while, others had vesicular nuclei and acidophilic cytoplasm; many cells were binucleated and slightly dilated blood sinusoids (Figure 8). The Portal area showed less dilated portal vein with marked reduction in cellular infiltration in comparison with ZnONPs-treated group (Figure 9).
Figure 8.A photomicrograph of a section in the liver of ZnONPs & VE- treated adult albino rat showing slightly dilated sinusoids (s) and central vein (Cv) with flat endothelial lining (thin arrows). Most of hepatocytes are with vesicular nuclei and acidophilic cytoplasm (H). Others show darkly-stained nuclei (h) and less vacuolated cytoplasm (v). Binucleated cells (thick arrows) are also seen. (H&E X400)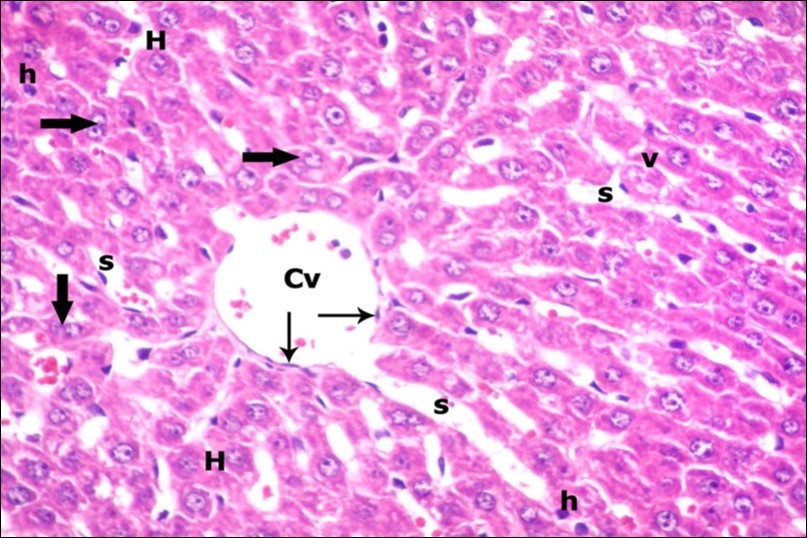
Figure 9.A photomicrograph of a section in the liver of ZnONPs & VE- treated adult albino rat showing portal area; portal vein (Pv) with flat endothelial lining (thin arrows), bile duct (Bd) and hepatic artery (Ha). Few inflammatory cells are observed (curved arrows). Most of hepatocytes with vesicular nuclei (H) show strong acidophilic cytoplasm (arrow head), some hepatocytes with darkly-stained nuclei (h) and vacuolated cytoplasm (v). Binucleated cells (thick arrows) are also seen. (H&E X400)
Masson’s trichrome-stained liver sections showed normal distribution of collagen fibers around the portal area in the 1st group (Figure 10a) and no difference could be detected in the 2nd group. In the 3rd group there was abundant increase in the amount of the blue-stained collagen fibers around the portal areas (Figure 10b). However, in the 4th group there was marked reduction in deposition of collagen fibers around the portal area compared to ZnONPs-treated group (Figure 10c).
Figure 10.Photomicrographs of sections in the liver: (a) Control group showing normal distribution of collagen fibers (arrow). (b) ZnONPs-treated group showing abundant stained collagen fibers surrounding the portal area. (c) ZnONPs &VE- treated group showing reduction of the collagen content in portal area. (Masson’s trichrome X 200)
Immunohisto Chemical Staining
Immunohistochemical stained sections of the 1st and 2nd groups showed negative immune reaction for caspase 3 inside the cytoplasm of hepatocytes (Figure 11a); while strong positive reaction for Caspase 3 were noticed in the 3rd group (Figure 11b); and in the 4th group the intensity of reaction was apparently decreased (Figure 11c).
Figure 11.Photomicrographs of sections in the liver: (a) Control adult albino rat showing negative immune reaction for caspase 3 inside the cytoplasm of hepatocytes (arrow). (b) ZnONPs-treated adult albino rat showing strong positive immune reaction for caspase 3 inside the cytoplasm of hepatocytes. (c) ZnONPs & VE-treated adult albino rat showing weak positive immune reaction for caspase 3 inside the cytoplasm of hepatocytes. (Immunoperoxidase technique for caspase 3 X 400)
Statistical Analysis of Morphometric Results
Animals body weights and liver weights revealed a significant decrease (p <0.001) in the ZnONPs-treated group when compared with other groups (Table 1, Table 2).
Table 1. Comparison between mean values of body weight (gm) in the different studied groups using ANOVA (analysis of variance) test.| Groups/Parameter | Control group(n=12) | VE group(n=12) | ZnONPs group(n=12) | ZnONPs+VE group(n=12) | F | P |
| Body weight:(gm)Mean ±SD | 184 ±6.5 | 182.2 ±5.0 | 148.8 ±5.3 | 179.8 ±6.7 | 74.2 | <0.001 |
| Groups/Parameter | Control group(n=12) | VEgroup(n=12) | ZnONPs group(n=12) | ZnONPs+VEgroup(n=12) | F | P |
| Liver weight: (mg)Mean ± SD | 7.5 ± 0.3 | 7.2 ± 0.2 | 5.1 ± 0.4 | 6.8 ± 0.4 | 81.17 | <0.001 |
The results of the present study showed a very highly significant increase (P<0.001) in the number of hepatocytes with positive immune reaction for Caspase 3 in the ZnONPs-treated group when compared with other groups (Figure 12).
Figure 12.The mean area percentages of caspase 3 expression in the different studied groups.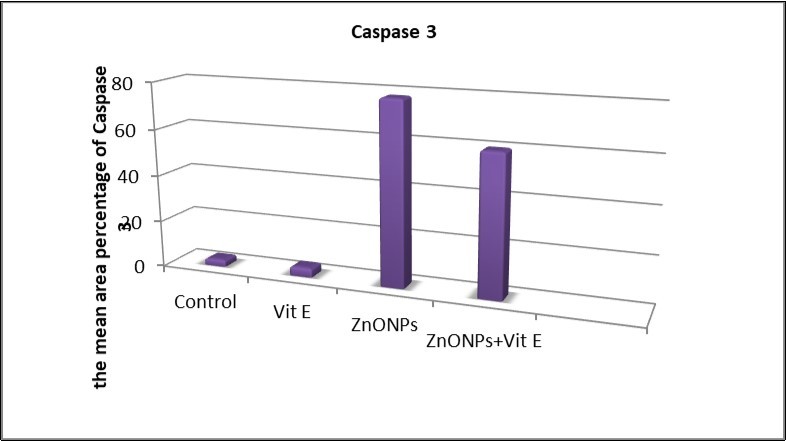
Regarding the collagen distribution, the results of the present study showed a very highly significant increase (P <0.001) in area of collagen in the ZnONPs-treated group when compared with other groups (Table 3).
Table 3. Showed that the mean area percentage of collage deposition in Masson trichrome stained sections in different studied groups| Groups/Parameter | Controlgroup(n=12) | VEgroup(n=12) | ZnONPsgroup(n=12) | ZnONPs+VEgroup(n=12) | F | P |
| Collagen: (Area%)Mean ±SD | 0.19 ±0.06 | 0.17 ±0.05 | 0.34 ±0.11 | 0.20 ±0.07 | 9.48 | <0.001 |
By using least significant difference (LSD) for comparison between means values of liver weight in between groups it was found that:
1st group vs. 2nd group: > 0.05 non-significant.
1st group vs. 3rd group : < 0.001 highly significant.
1st group vs. 4th group: > 0.05 non-significant.
3rd group vs. 4th group: < 0.001 highly significant.
Biochemical Results
Regarding the liver enzymes, there was very significant increase in serum activities of ALT and AST (P<0.001) in the ZnONPs-treated group when compared with other groups (Table 4; Figure 13).
Table 4. Comparisons between mean values of ALT & AST in the different studied groups using ANOVA test| Groups/Parameter | Controlgroup(n=12) | VEgroup(n=12) | ZnONPsgroup(n=12) | ZnONPs+VEgroup(n=12) | F | P |
| ALT: (U/L)Mean ±SD | 44.2 ± 3.9 | 46.8 ± 10.4 | 158 ± 12.1 | 51.6 ± 8.7 | 316.19 | <0.001 |
| AST: (U/L)Mean ± SD | 119.1 ± 12.5 | 121.3 ± 17.6 | 240.1 ± 13.2 | 124.1 ± 18.2 | 154.53 | <0.001 |
Figure 13.Comparison between mean values of ALT & AST in different studied groups using ANOVA (analysis of variance) test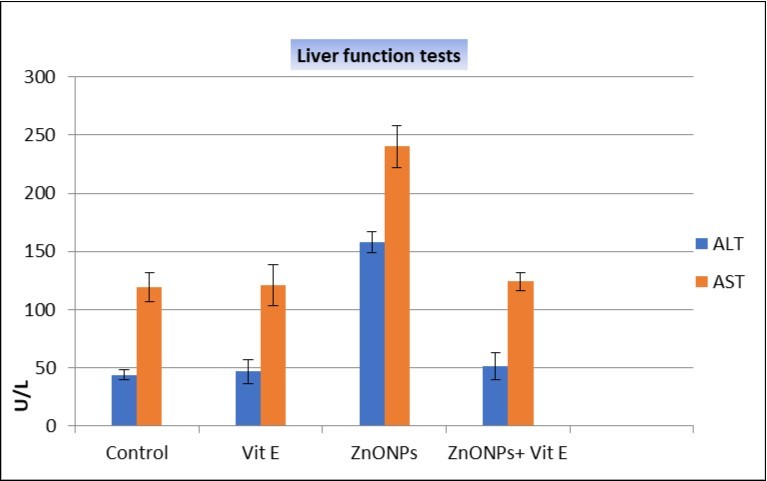
Discussion
It has been documented that the tissue structure and biochemical changes as well as the body and organ weights are the determinant factors in evaluating the toxicity of nanoparticles 26. In this study, we investigated the liver structure and some enzymes in animals exposed to ZnONPs to determine such toxicity. Adult albino rats were selected in the current study as they have relatively long life span free from diseases and they are easily handled. In addition, male animals were favored as they have relatively constant hormone levels which help to pass over the role that could such hormones play in many inflammatory conditions 27.
Regarding the weights of the animals and their livers, it was noticed that marked reduction occurred in ZnONPs exposed animals that relatively improved with administration of VE. These findings are in the line with previous study 19. This is an indication of toxicological effect in ZnONPs-treated animals.
In the present work, H&E-stained liver sections of the ZnONPs-treated group showed disruption of normal architecture with congestion and dilatation of central as well as blood sinusoids. These results are in accordance with Johar et al., 28 who suggested that the destruction of lobular structure, vacuolization of hepatocytes (fat deposits), and infiltration of leukocytes indicate necrotic effects of ZnONPs on liver tissue. On the other hand, Puche et al., 29 attributed sinusoidal dilatation to the activation of perisinusoidal cells which had contractile properties. On contrary to the current results, Wang et al 26 found minimal toxicity through their biodistribution assays in liver tissue of mice exposed to 50 and 500 mg/kg ZnONPs.
Some hepatocytes appeared ballooned with dark stained nuclei. This finding is in consistence with Abbasalipourkabir et al., 30 who gave 50, 100, 150 and 200 mg/kg ZnONPs attributed ballooning of hepatocytes to apoptosis induced by ZnONPs. Ma et al. 31 revealed that swelling of hepatocytes indicates that these nanoparticles may affect permeability of the cell membrane in hepatocytes. Al-Rasheed et al., 13 stated that the liver of rats treated with a high dose of ZnONPs showed numerous hepatocytes with karyolysis and pyknotic nuclei in addition to inflammatory cellular infiltration.
Foci of cellular infiltration were detected in the liver parenchyma. Such result is parallel with Sharma et al., 14 who stated that the liver revealed hepatocellular necrosis and accumulation of mixed inflammatory cells around the necrotic area. Edinger and Thompson 32 attributed the presence of necrotic foci to lysis of cells and formation of cell debris, which could initiate phagocytic infiltration. In the current study the portal area revealed portal vein dilatation and congestion. Hu et al. 33 explained that the congestion and dilatation of central and portal veins may be due to portal hypertension. Bile duct proliferation was evident in the current ZnONPs- treated group. This result is in agreement with Pati et al. 34 who documented that a ductular reaction is the proliferative response to many types of liver injuries. It is now generally accepted that the liver contains hepatic stem cells/ progenitor cells in which are considered as a subpopulation of liver cells termed oval cells. Under the condition of severe and chronic liver injury caused by drugs, toxins and viruses, these oval cells are induced to proliferate and differentiate both into biliary epithelial cells and into mature hepatocytes 35.
In the current study, portal veins of rat treated with ZnONPs revealed elongation of their endothelial lining. Wen et al. 36 attributed this result to smooth muscle cell (SMC) proliferation and hypertrophy in the portal vein. They indirectly proved that nitric oxide (NO) is closely related with portal venous vascular remodeling that will lead to SMC proliferation and hypertrophy. Also, the wall of hepatic artery showed increasing of its muscular wall. Reneman et al., 37 explained this result to that Shear stress can promote remodeling of the arterial walls, possibly through regulation of endothelial cells (EC) gene expression and intracellular bioactive substances.
In the 4th group, there was some improvement of liver architecture when compared with group III. Preservation of normal structures in the protected groups was attributed to the anti-inflammatory role of VE which associated with the amelioration of oxidative damage 38. The binucleated hepatocytes seen the ZnONPs+VE treated groups may be due to the regenerative attempt of the degeneration of cells as reported earlier in the liver of carbaryl treated rats by Munglang et al., 39.
Regarding Masson’s trichrome-stained sections, there was marked increase collagen fibers around the portal area in ZnONPs- treated group. These results are in agreement with that of Al-Rasheed et al., 13. Galli et al., 40 stated that ROS are incriminated in the development of hepatic fibrosis as they stimulate hepatic stellate cells (HSCs) proliferation and collagen synthesis.Hepatic stellate cells which are relatively inactive fibroblasts in liver lobules play an important role in liver fibrosis. Apparent reduction in collagen fibers content around the portal area was obvious in group IV.
Caspase 3 stained liver sections of ZnONPs- treated rats showed strong positive reactions in the cytoplasm of hepatocytes. These findings are in accordance with those of Yousef and Mohamed 41, who stated that the liver apoptosis biomarker caspase3 was significantly up-regulated in rats administered either ZnO-bulk or its NPs orally (500 mg/kg bw) for 10 successive days. These findings confirmed statistically by morphometric analysis of the number of hepatocytes with caspase 3 positive immunoreaction and area percentage of collagen fiber that showed a highly significant increase (P<0.001) in ZnONPs- treated group when compared with that of the control group. In addition, there was a non-significant difference between the control and ZnONPs+VE- treated groups, this is in accordance with Al-Rasheed et al., 13.
In the present study, both liver and body weights showed highly significant decrease in the ZnONPs- treated group when compared with the control groups. However, no significant difference was observed between the control animals and both VE and ZnONPs plus VE- treated groups. This result is in accordance with that of Ko et al. 42. However, Sharma et al., 14 observed that there was no significant difference in the body weights between control and ZnONPs- treated groups. Similarly, no obvious difference was observed in the organ weights.
On the other hand, the changes in ALT and AST levels were statistically decreased in ZnONPs+VE treated group when compared with ZnONPs animals. This result is in agreement with Al-Rasheed et al. 13 who reported that VE has the ability to normalize levels of such enzymes.
It was concluded that ZnONPs might induce some histological and biochemical adverse effects on liver of adult male albino; and VE alleviated most of these changes. Eventually, the study suggests the use of VE as a protective approach against the toxic effects of ZnONPs. Future studies using large numbers and different models of animals are recommended with follow up of the animals for longer period to investigate the recovery from such adverse effects.
Abbreviations
ALT: Alanine transaminase
ANOVA: Analysis of variance
AST: Aspartate transaminase
H&E: Hematoxylin and eosin
LM: Light microscopy
LSD: Least significant difference
ROS: Reactive oxygen species
SD: Standard deviation
VE: Vitamin E
ZnONPs: Zinc oxide nanoparticles
Funding
None
References
- 1.Mescher A. (2013) Junqueira's Basic Histology: Text and Atlas. (13th Ed.). McGraw-Hill Education. Pp: 329-339.
- 2.Nesseem D.Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. , Int. J. Cosmet. Sci.,2010 33(1), 70-79.
- 3.Bai H, Liu Z, Sun D D.Hierarchically multifunctional TiO2 NPs modification for water purification. , Chem. Commun.,2010,46: 6542, 6544.
- 4.Jones N, Ray B, Ranjit K T, Manna A C. (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. , FEMS Microbiol. Lett 279(1), 71-76.
- 5.Tsuji J S, Maynard A D, Howard P C, James J T, Lam C W. (2006) Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicological sciences. 89(1), 42-50.
- 6.Møller P, Jacobsen N R, Folkmann J K, Danielsen P H, Mikkelsen L. (2010) Role of oxidative damage in toxicity of particulates. Free radical research. 44(1), 1-46.
- 7.Luo J, Borgens R, Shi R.Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. , J. of Neurochem.,2002,83(2): 471-480.
- 8.Manke A, Wang L, Rojanasakul Y.Mechanisms of nanoparticle-induced oxidative stress and toxicity. , Bio. Med. Res.,2013,2013: 1-15.
- 9.Baek M, Chung H E, Yu J, Lee J A, Kim T H. (2012) Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. , Int. J. Nanomedicine,7: 3081-3097.
- 10.Landsiedel R, Ma-Hock L, Kroll A. (2010) Testing metal-oxide nanomaterials for human safety. , Adv. Mater 22(24), 2601-2627.
- 11.Buzea C, Pacheco I, Robbie K. (2007) Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2(4), 18-65.
- 12.John E, Laskow T C, Buchser W J, Pitt B R, Basse P H. (2010) Zinc in innate and adaptive tumor immunity. , J. Trans. Med 8(1), 118-120.
- 13.Al-Rasheed N M, Baky N A, Faddah L M, Fatani A J, Hasan I H. (2014) Prophylactic role of a-lipoic acid and vitamin E against zinc oxide nanoparticles induced metabolic and immune disorders in rat’s liver. Eur Rev Med Pharmacol Sci. 18(12), 1813-1828.
- 14.Sharma V, Singh P, Pandey A K, Dhawan A. (2012) Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 745(1), 84-91.
- 15.Bakhshiani S, Fazilati M. (2014) Vitamin C can reduce toxic effects of Nano Zinc Oxide. , Int. Res. J. Biol. Sci 3(3), 65-70.
- 16.Ziani K, Fang Y, McClements D J. (2012) Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 134(2), 1106-1112.
- 17.Biesalski H K. (2009) Vitamin E requirements in Parenteral Nutrition Gastroenterology,137(5):S92–S104.
- 18.Shati A A.Ameliorative effect of vitamin E on potassium dichromate-induced hepatotoxicity in rats. , Journal of King Saud University-Science,2014 26(3), 181-189.
- 19.Wang B, Feng W Y, Wang M, Wang T C, Gu Y Q. (2008) Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. , Journal of Nanoparticle Research 10(2), 263-276.
- 20.Sharma M, Gupta Y K. (2003) Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur Neuropsychopharmacol. 13(4), 241-247.
- 21.Hegazy R.Hegazy A.): Hegazy’ Simplified Method of Tissue Processing (Consuming Less Time and Chemicals) Ann. , of Int. Med. & Den. Res.2015 1(2), 57-61.
- 22.Bancroft J, Gamble M. (2008) Theory and practice of Histological technique (6thEd.).Churchill. , Livingston, New York, Edinburgh, London. Pp: 165-175.
- 23.Krajewska M, Wang H G, Krajewski S, Shabaik A, Gascoyne R. (1997) Immunohistochemical analysis of in vivo pattern of expression of CPP32 (Caspase 3), a cell death protease. Cancer Res. 57(8), 1605-1631.
- 24.Reitman S, Frankel S. (1957) A colometric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. , Am. J. Clin. Pathol 28(1), 56-63.
- 25.Kirkwood B R, JAC Sterne. (2003) Essential medical statistics. BlackwellScience,Inc,350 , Main Street, Malden, Massachusetts02148-5020,USA:Blackwell.ISBN978-086542-871-3 .
- 26.Wang C, Lu J, Zhou L, Li J, Xu J et al. (2016) . Effects of Long-Term Exposure to Zinc Oxide Nanoparticles on Development, Zinc Metabolism and Biodistribution of Minerals (Zn, Fe, Cu, Mn) in Mice.PLoSONE,11(10):e0164434 .
- 27.Hegazy A A, Elsayed N E, Ahmad M M, Omar N M. (2017) . , Effect of Formaldehyde on Rat Testis Structure. Acad. Anat. Int 3(2), 15-23.
- 28.Johar D, Roth J C, Bay G H, Walker J N, Kroczak T J. (2004) Inflammatory response, reactive oxygen species, programmed (necrotic-like and apoptotic) cell death and cancer. Roczniki Akademii Medycznej w Bialymstoku. 1995(49), 31-39.
- 29.Puche J, Saiman Y, Friedman S. (2013) Hepatic Stellate Cells and Liver Fibrosis. Compr Physiol. 3, 1473-1492.
- 30.Abbasalipourkabir R, Moradi H, Zarei S, Asadi S, Salehzadeh A. (2015) Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food and Chemical Toxicology. 84, 154-160.
- 31.Ma L, Zhao J, YM Wang J Duan, Liu J. (2009) The acute liver injury in mice caused by nano-anatase TiO2. Nanoscale research letters. 4(11), 1275-1285.
- 32.Edinger A L, Thompson C B. (2004) Death by design: apoptosis, necrosis and autophagy. Current opinion in cell biology. 16(6), 663-669.
- 33.Hu L S, George J, Wang J H. (2013) Current concepts on the role of nitric oxide in portal hypertension. , World journal of gastroenterology.World J Gastroenterol,19 11, 1707-1717.
- 34.Pati R, Das I, Mehta R K, Sahu R, Sonawane A. (2016) Zinc-oxide nanoparticles exhibit genotoxic, clastogenic, cytotoxic and actin depolymerization effects by inducing oxidative stress responses in macrophages and adult mice. Toxicological Sciences. 150(2), 454-472.
- 36.Wen B, Liang J, Deng X, Chen R, Peng P.Effect of fluid shear stress on portal vein remodeling in a rat model of portal hypertension. Gastroenterology research and practice. 2015(545018), 1-7.
- 37.Reneman R S, Arts T, Hoeks A P. (2006) Wall shear stress–an important determinant of endothelial cell function and structure–in the arterial system in vivo. Discrepancies with theory, J Vasc Res 43(3), 251-269.
- 38.Qureshi A A, Reis J C, Qureshi N, Papasian C J, Morrison D C et al. (2011) δ-Tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids in Health and Disease 10(1), 39-58.
- 39.Munglang M, Nagar M, Prakash R. (2009) Liver in carbaryl treated rats–a morphological and morphometric study. , India, J. Anat. Soc 58(1), 6-9.
- 40.Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F et al. (2005) Oxidative Stress Stimulates Proliferation and Invasiveness of Hepatic Stellate Cells via a MMP2- Mediated Mechanism. Hepatology. 41(5), 1074-1084.
Cited by (12)
- 1.Sakr Samar, Steenkamp Vanessa, 2021, Zinc oxide nanoparticles induce oxidative stress and histopathological toxicity in the thyroid gland and liver of rats, Toxicological & Environmental Chemistry, 103(4), 399, 10.1080/02772248.2021.1941021
- 2.Khanam Sabina, 2020, Toxicological effect of zinc on liver of broiler chicks, Egyptian Liver Journal, 10(1), 10.1186/s43066-020-00028-w
- 3.Chong Ce Lynn, Fang Chee Mun, Pung Swee Yong, Ong Chin Eng, Pung Yuh Fen, et al, 2021, Current Updates On the In vivo Assessment of Zinc Oxide Nanoparticles Toxicity Using Animal Models, BioNanoScience, 11(2), 590, 10.1007/s12668-021-00845-2
- 4.Moatamed Eman Raafat, Hussein Aida Ahmed, El-desoky Mohamed Mahmoud, Khayat Zakaria EL, 2019, Comparative study of zinc oxide nanoparticles and its bulk form on liver function of Wistar rat, Toxicology and Industrial Health, 35(10), 627, 10.1177/0748233719878970
- 5.Mohamed Mowafy Sarah, Awad Hegazy Abdelmonem, A. Mandour Dalia, Salah Abd El-Fatah Samaa, 2021, Impact of copper oxide nanoparticles on the cerebral cortex of adult male albino rats and the potential protective role of crocin, Ultrastructural Pathology, 45(4-5), 307, 10.1080/01913123.2021.1970660
- 6.Al-Ali Ali A. A., Al-Tamimi Shatha Q., Al-Maliki Sami J., Abdullah Mohd Azmuddin, 2022, Toxic effects of zinc oxide nanoparticles and histopathological and caspase-9 expression changes in the liver and lung tissues of male mice model, Applied Nanoscience, 12(2), 193, 10.1007/s13204-021-02248-x
- 7.Wazir Noman Ullah, Khattak Tania, Khan Usama, Hussain Saman, Shah Syed Mohammad Tahir, et al, 2024, Unveiling the Therapeutic Potential of Vitamin-E in Preventing Inflammation and Stromal Congestion in Alcoholic Liver Injury, Cureus, (), 10.7759/cureus.54931
- 8.Al-Azhary Diaa B, Sawy Samar A, Fawzy Hassan Hanaa, Meligi Noha M, 2023, Potential effects of spirulina and date palm pollens on zinc oxide nanoparticles -induced hepatoxicity, oxidative stress, and inflammation in male albino rats, Toxicology Research, 12(6), 1051, 10.1093/toxres/tfad096
- 9.Kausar Sana, Jabeen Farhat, Latif Muhammad Asif, Asad Muhammad, 2023, Characterization, dose dependent assessment of hepatorenal oxidative stress, hematological parameters and histopathological divulging of the hepatic damages induced by Zinc oxide nanoparticles (ZnO-NPs) in adult male Sprague Dawley rats, Saudi Journal of Biological Sciences, 30(9), 103745, 10.1016/j.sjbs.2023.103745
- 10.Umeaku Ugochukwu, Ofusori David A., Umeaku Obioma P., Edward Tolulope A., Hegazy Ahmed M S, 2020, The Effect of Aqueous Extract of Ocimum gratissium (Linn) on 1, 2 - Dimethyl Hydrazine induced Colon Cancer in Male Wistar Rats, International Journal of Human Anatomy, 2(1), 22, 10.14302/issn.2577-2279.ijha-20-3180
- 11.Anderson Enye Linus, Angel Keboh, Edem Edem, Olusola S Saka, Godson Akunna Gabriel, et al, 2020, Histological and Biochemical Study on Mitigation of Dichlorvos-Induced Hepatotoxicity by Mimosa Pudica in Mice, International Journal of Human Anatomy, 2(2), 15, 10.14302/issn.2577-2279.ijha-20-3232
- 12.Abdelnaem Aya M., Fathy Hala, Yahia Doha, Ali Marwa F., Nassar Ahmed Y., et al, 2024, Ameliorative effects of Copper(II) albumin complex against zinc oxide nanoparticles induced oxidative DNA damage in Sprague Dawley rats, Toxicology and Environmental Health Sciences, (), 10.1007/s13530-024-00208-w
