Abstract
Since oxidative stress impairs the cardiovascular function, the hypothesis from the present study is that the treatment of paraquat-exposed adult Wistar rats with methanolic extract of Abelmoschus esculentusseed would reduce paraquat-induced cardiovascular damage.
Thirty healthy female Wistar rats weighing 120-150 g were randomly assigned into 6 groups of 5 rats each (Groups A, B, C, D, E and F). Rats in groups A served as control and received normal saline while groups B, C, D, E and F received a single dose of paraquat (7mgkg-1i.p.). Rats in group B was sacrificed 24hours following paraquat administration while daily administration of 100 mg kg-1and 200 mg kg-1 of methanolic extract of Abelmoschus esculentusseed extract were given orally to groups C and D while group E received daily oral dose of Vitamin E at 100mgkg-1 and group F was left untreated. Histological and biochemical preparations of the heart was made and data were expressed as mean± SEM. Significant difference was set at p<0.05. Results showed no significance difference (p<0.05) in nitric oxide activity, Glutathione reductase activity, and troponin I activity across the paraquat-exposed groups when compared with control. Histological studies reveal distortion of normal cardiac histo-architecture in paraquat-exposed group B compared with control rats while Abelmoschus esculentus reversed these changes in other treated groups. The study concluded that paraquat caused significant distortion of the cardiac histo-architecture and methanolic extract of immature Abelmoschus esculentus seed had antioxidant and ameliorative effects similar to Vitamin E on paraquat-induced myocardial injury.
Author Contributions
Academic Editor: Abdelmonem Awad Mustafa Hegazy, Professor and Former Chairman of Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Anjorin O. Atinuke, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Herbicides have been used in conjunction with host plant resistance, cultural, mechanical, and biological tactics in an integrated pest management system to combat the battle against destructive pests 1. The American Heritage Dictionary defines herbicide as "a chemical that is used to control weeds ".1.
Paraquat is the trade name for N,N′-dimethyl-4,4′-bipyridinium dichloride, the organic compound with the formula C12H14Cl2N2 2. It is toxic to human beings and animals. It has been linked to development of Parkinson's disease 3. Paraquat can be absorbed into the body by inhalation of its aerosol, through the skin and or eye contact and by ingestion 4. Previous studies on rats has demonstrated that administration or accidental ingestion of paraquat dichloride causes an extremely high fatality rate of between 30–70% 5, 6
Either direct toxicity through oxidative stress or indirect route through ROS accumulation and activation of pro-inflammatory stress signals/cytokines can contribute to paraquat-induced toxicity and organ injury 7.
Cardiovascular diseases are a major cause of premature mortality in both the developed and developing world. A number of risk factors which are associated with cardiovascular disease may be linked, at least in part, to oxidative stress 8. Oxidative stress can also lead to dysfunction in endothelial cells, monocytes, vascular smooth muscle cells and mitochondrial damage 9, 10, 11, 12, 13.
Abelmoschus esculentus (Okra) is a flowering plant, one of the members of the Malvaceae (mallow) family. It had been established that okra is rich in antioxidants and phenolic compounds and reduces the risk of cardiovascular diseases, stroke and certain forms of malignancies 14. On the other hand, oxidative stress is intricately linked with ageing related diseases (ARDs) and longevity 15. The metabolism of phospholipids is in fact associated with neuronal death in Alzheimer’s disease (AD) and antioxidants such as vitamin E play an important role in ß-amyloid (Aß) aggregation 16, while the Cache County Study demonstrated that the use of vitamin E and vitamin C supplements in combination is associated with a reduced prevalence and incidence of Alzheimer’s disease (AD) 17. A role for ceramide in inducible nitric oxide synthase induction and Nitric Oxide (NO) production in AD pathobiology and provided a possible explanation for the beneficial effects of vitamin E therapy in AD patients 18, 19.
Since oxidative stress impairs the cardiovascular function, the hypothesis from the present study is that the treatment of paraquat-exposed adult Wistar rats with methanolic extract of Abelmoschus esculentusseed would reduce paraquat-induced cardiovascular damage. Hence, this study aims to look at the effect of Okra on the heart of Wistar rats, following paraquat exposure.
Materials and Methods
Experimental Design
Thirty female Wistar rats weighting between 120g-150g obtained from Animal Holdings of Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ile-Ife were used for this research. The rats were assigned into six groups of five animals each. Group A was the control Rat in this group received of 0.3 ml of saline, group B was positive control /acute toxic group, groups C, D and E were the test groups and group F was the recovery group. Rats in groups B, C, D, E and F were given paraquat at 7mgkg-1i.p as a single dose in addition, groups C and D were given daily administration of 100 mg kg-1 and 200 mg kg-1 of methanolic extract of Abelmoschus esculentus seed (MEAS) orally while group E was given 100 mg kg-1 Vitamin E orally. Group B was sacrificed 24 hours following paraquat administration while group F was left for 3 weeks before sacrifice. The extract solution and Vitamin E were administered orally, using oral cannula while paraquat was administered intraperitoneally. The duration of the experiment was 3 weeks.
Animal Care and Management
Ethical Clearance for the study was obtained from Health Research Ethical Committee (HREC), Institute of Public Health (IPH), Obafemi Awolowo University (OAU), Ile-Ife (IPH/OAU/12/705). The animals were housed in Animal Holdings of Basic Medical Sciences, Obafemi Awolowo University, Ile-Ife. They were maintained on standard laboratory pellet in natural day and night cycle and and clean water was provided ad libitum.
Plant Material and Preparation of Extract
Fresh immature Okra (Abelmoschus esculentus) fruits were purchased from a market in Ile-Ife in Osun State, Nigeria. The leaves and fruits were authenticated by a taxonomist in the Department of BotanyObafemi Awolowo University, Ile-Ife; the Abelmoschus esculentus fruits were thoroughly cleaned with water. After that, the seeds and pulp were separated and the seeds were roasted at 60ᵒC and powdered in a warring blender. 370g of powdered seed was extracted in 1000mlsof 80% v/v methanol while sonicating for 72 hours, it was then filtered and the filtrate was concentrated in vacuo at 30°C under reduced pressure in a vacuum rotary evaporator (Buchi Rotavapor R110, Schweiz) until a crude solid extract was obtained which was freeze-dried for complete solvent removal. It was then stored in a desiccator till use. The stock solution was prepared by dissolving 10gms of the extract in 100ml of normal saline. The volume of the stock given to each animal was calculated using the modified formula below; Volume (ml) =dose of extract (mg.kg-1) x weight of the rat (kg)/concentration of stock (mg/ml)
Drug Administration
Paraquat was diluted in 0.9% saline and administered at a single dose of 7mg /kg body weight intraperitoneally as determined by the lethal dose.
Vitamin E was purchased with the Trade name –EVIOL (GAP S. A, Athen- Greece) from a pharmacy shop in Ile-Ife, Osun State, Nigeria. It was administered orally at 100mg/kg daily for 3 weeks to group E rats 8.
Preparation of Paraquat
Paraquat (dichloride-1, 1-dimethyl-4, 4-bipyridynium) was purchased from a chemical store in Ile-Ife as 200g paraquat ion per litre solution, with the trade name of‘ Weed Crusher’ (Alderelm Limited, United Kingdom), properly sealed in transparent plastic container. The dose of paraquat (30% LD50) was calculated and stock solution was prepared accordingly by dilution with normal saline. The LD50 of paraquat was determined by the method of Lorke (1983) as modified by Imafidon et al.,20.
Phytochemical Screening of MEAS
The method was described to test for the presence of phytochemical in the extract 21.
Sacrifice of Animals
Twenty four hours following the final administration, rats were sacrificed through cervical dislocation. Blood samples were collected from each animal through cardiac puncture into sample bottles with the aid of non-heparized capillary tubes. The blood samples were centrifuged at 2000 rpm for 20 minutes and blood sera were collected and stored at 40C. Serum was frozen until the biochemical determination was performed. A midline incision was made through the thorax, the heart was excised and tissue from the left ventricle was separated by a transverse section through the midpoint between the apex and the base of the heart.
Determination of Body Weight and Heart Weight
Body weights were taken at the beginning of the experiment and weekly before sacrifice. Blood was obtained from the retro-orbital sinus. Animals were sacrificed 24 hours following the last administration. After sacrifice, the thorax was dissected through a midline incision, the heart was harvested and weighed using the Metler Toledo P163 sensitive weighing balance and fixed in 10% formal-saline for histological studies.
Relative organ weight was calculated using the formula;
Organ weight/Final body weight
Percentage Weight Change was Calculated Using
Final weight-Initial weight/Initial weight x 100
Histological Stains
The excised heart tissue was fixed in 10% formal saline for 48 hours, and processed using paraffin wax embedding method. Sections of 5 μm thickness were cut from the paraffin embedded tissues and stained with haematoxylin and eosin stain to demonstrate the general histo-architecture of the left ventricle, Verhoeff-Van Gieson stain was used to demonstrate elastic fibers in the left ventricle and Toluidine blue was used to demonstrate mast cells.
Verhoeff – Van Gieson Stain
Slides were brought to distilled water, stained in Verhoeff’s solution for an hour thereby rendering tissue black coloration then rinsed in tap water with 2 – 3 changes and differentiated in 2% ferric chloride solution for 1 – 2 minutes. Therefore, slides were washed in rap water, treated for a minute in 5% sodium thiosulphate solution after which the solution was discarded and washed for 5 minutes in running tap water. Counterstained in Van Gieson’s solution for 3 – 5 minutes and dehydrated quickly through 95% alcohol for 2 changes of absolute alcohol which later cleared in 2 changes of xylene for 3 minutes each and finally cover-slipped with resinous mounting medium.
Toluidine Blue
This was used to demonstrate mast cells which are found widely distributed in the connective tissue. Their cytoplasm contains granules composed of heparin and histamine which are metachromatic. Sections were deparaffinised, hydrated in distilled water and stained in working Toluidine blue stain for 2 minutes. It was rinsed in 3 changes of distilled water, dehydrated rapidly in 95% and absolute alcohols. It was cleared in xylene and Cover-slipped using an aqueous mountant.
Enzymatic Assay
Determination of Nitric Oxide Levels in Serum
At the end of the experiment, the serum was separated from the blood by centrifugation at 3,000g for 10 min. The serum NO levels were determined using Nitric oxide assay kit for the colorimetric determination of total nitrite (Bioassays Systems, USA).
Glutathione Reductase Activity (GR)
GR activity was assayed by the method of Sharma et al., 2001. The assay mixture consisted of 1.6 ml of sodium phosphate buffer (0.1 M pH 7.4), 0.1 ml EDTA (1mM), 0.1 ml of 1mM oxidized glutathione, 0.1ml of NADPH (0.02mM), 0.01 ml of 1mM H2O2 and 0.1 ml PMS in a total volume of 2 ml. The enzyme activity measured at 340 nm was calculated as nmoles of NADPH oxidized/min/mg of protein using values of 6.22 × 103 M-1 cm-1.
Cardiac Troponin I Assay (CTNI)
The desired number of coated wells was secured in the holder.100 ml of cTnI horseradish peroxidase (HRP) Conjugate was dispensed into each well.100 ml of standards and samples was dispensed into the appropriate wells. This was thoroughly mixed for 10-15 seconds. This was incubated on an orbital shaker (150 rpm) at room temperature (18-25°C) for 60 minutes. The incubation mixture was removed by flicking the plate contents into a bio-waste container. The microtiter wells was washed and emptied 5 times with 1 wash solution. This was performed using plate washer (400 ml/well). The entire wash procedure was carried out.
Cytokeratin – 18 Assay (CK-18)
The different concentration of Rat CK-18 standard samples were added to the corresponding wells (100μl for each well). Blank wells were filled with standard diluents and the reaction wells were sealed with adhesive tapes and hatched in an incubator at 37°Cfor 90 minutes. Firstly, the Elisa plate was washed 3 times and the biotinylated rat CK-18 antibody liquid was added to each well (100μl for each) and the reaction wells were sealed with adhesive tapes and hatching was done in the incubator at 37°C for 60 minutes. Secondly, Elisa plate was washed 3 times Enzyme-conjugate liquid was added to each well except the blank wells (100μl for each) and the reaction wells were therefore sealed with adhesive tapes and hatching was done in the incubator at 37°C for 30 minutes. However thirdly, Elisa plate was washed 5 times and 100μl colour reagent liquid was added to individual well (also into blank well) and hatched in the dark incubator at 37°C. The colour for high concentration of standard curve got very darker, gradient appeared and hatching was stopped. The chromogenic reaction was controlled within 30 minutes and 100μl colour reagent C was added to individual well (also into blank well) and properly mixed. Thereafter, the OD(450nm)was read within 10 minutes.
Photomicrography
Stained sections were viewed under a Leica DM750 microscope (Leica Microsystems, Heerbrugg, Switzerland) with digital camera attached (Leica ICC50) and digital photomicrographs were taken at various magnifications.
Statistical Analysis
One-way ANOVA was used to analyze data, followed by Student Newman-Keuls test for multiple comparisons. GraphPad Prism 5 (Version 5.03; Graphpad Software Inc., San Diego, CA) was the statistical package used for data analysis. Statistically significant difference was set at p<0.05.
Results
Phytochemical Screening of MEAS
Phytochemical screening of methanolic extract of Abelmoscus esculentus seeds in this study revealed the presence of Tannins, glycosides, resins, saponins, flavonoids, sterols, phenols, carbohydrates and alkaloids, while terpenoids and phlobatannins were absent (Table 1)
Table 1. Phytochemical ScreeningIntraperitonealLD50 of Paraquat
Intraperitoneal LD50 of paraquat (PARQ) was determined to be 22.63 mg/kg ≈ 23 mg/kg in adult Wistar rats.
Effect of MEAS on Percentage Body Weight of Paraquat-Exposed Rat Heart
There was no significant difference in percentage body weight change across all experimental groups (p<0.05) (Figure 1).
Figure 1.Effect of MEAS on Body Weight of paraquat-exposed rat. n = 5, values are expressed as % weight change ± SEM at p<0.05.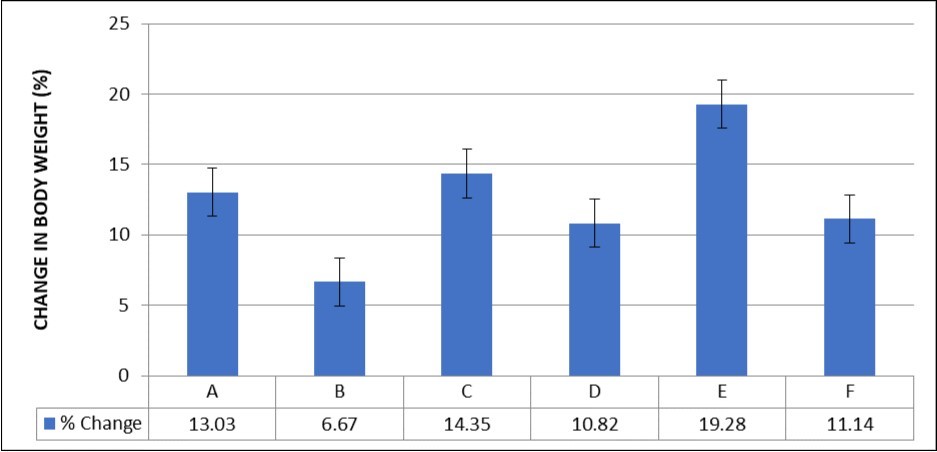
Haematoxylin and Eosin Staining
The hearts of control rats show the regular arrangement with clear striation of myocardial fibers without histological alterations, showing an outline of the normal heart. In group B, the cross-banding pattern of cardiac cells was distorted. In the group C, 100 mg kg-1 of methanolic extract of Abelmoscus esculentus seeds (MEAS) didn’t reverse the effect. In group D and group E, 200 mg kg-1 of MEAS and 100 mg kg-1 of Vitamin E the effect was reversed. However, in recovery group F, we also observed distortion of the myocardial fibres similar to group B (Figure 2).
Figure 2.Effect of paraquat exposure on the histo-architecture of the rat heart (A) Control, (B) Paraquat only, (C) MEAS 100mgkg-1, (D) MEAS 200mgkg-1, (E) Vitamin E 100mgkg-1, (F) Paraquat 7mg/kg -Recovery . H&E (x400)
Voerhoeff Van Gieson staining of control rats with the vessel as internal control shows abundant collagen and elastic fibres with a regular orientation which are found in the normal heart. In group B, there is loss of elastic fibres and distortion of collagen fibre. In plate C, there is some elastic fibre deposit and remodeling of collagen fibres. In plate D and plate E, 200 mg kg-1 of MEAS and 100 mg kg-1 of vitamin E reversal of the effect was noted as well as abundance of collagen and elastic fibres. Group F showed similar pattern to group C (Figure 3).
Figure 3.Effect of paraquat exposure on the elastic and collagen fibre distribution of the rat heart (A) Control, (B) Paraquat only, (C) MEAS 100mgkg-1, (D) MEAS 200mgkg-1, (E) Vitamin E 100mgkg-1, (F) Recovery group. VVG (x100)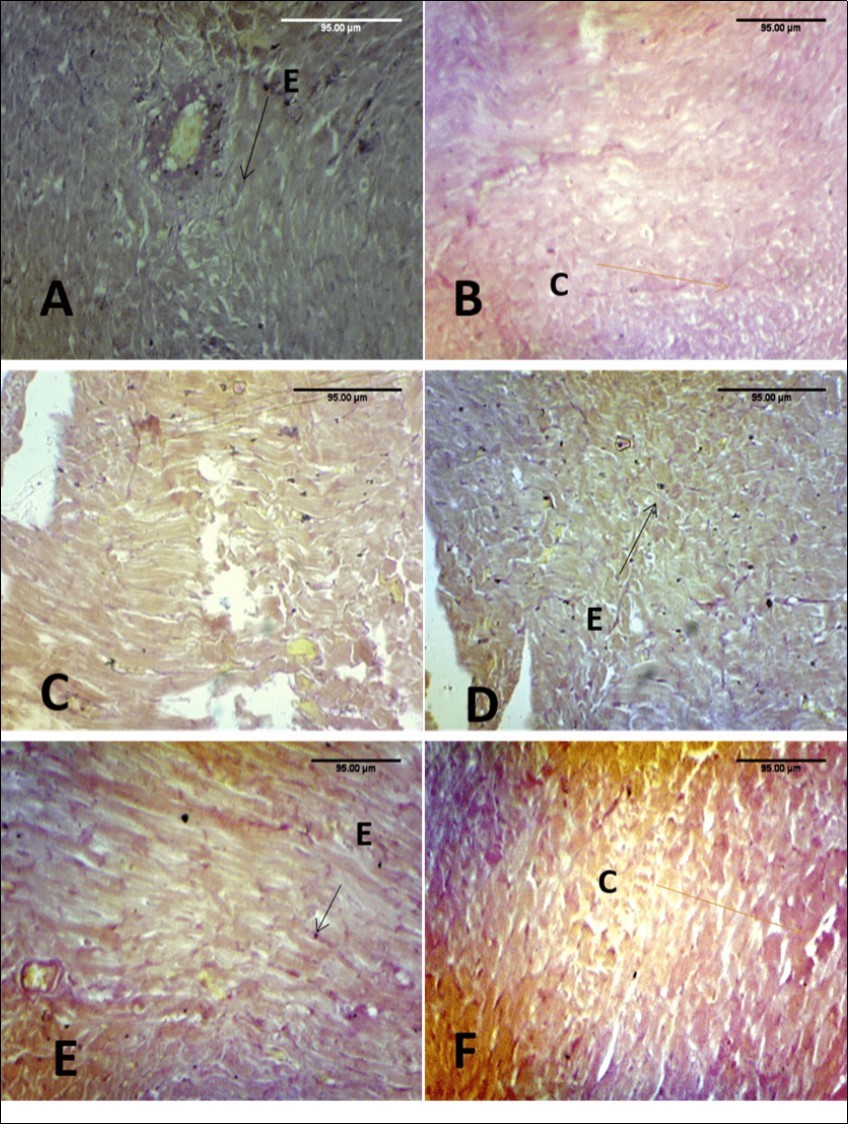
Toluidine blue staining group F showed abundant mast cells as well as group E. In group C (100 mg kg-1) little mast cells were seen and no mast cells in group D (200 mg kg-1of MEAS) (Figure 4).
Figure 4.Effect of paraquat exposure on mast cell distribution of the rat heart(A) Control, (B) Paraquat only (C) MEAS 100mgkg-1 (D) MEAS 200mgkg-1 (E) Vitamin E 100mgkg-1, (F) Recovery group. TB(x40)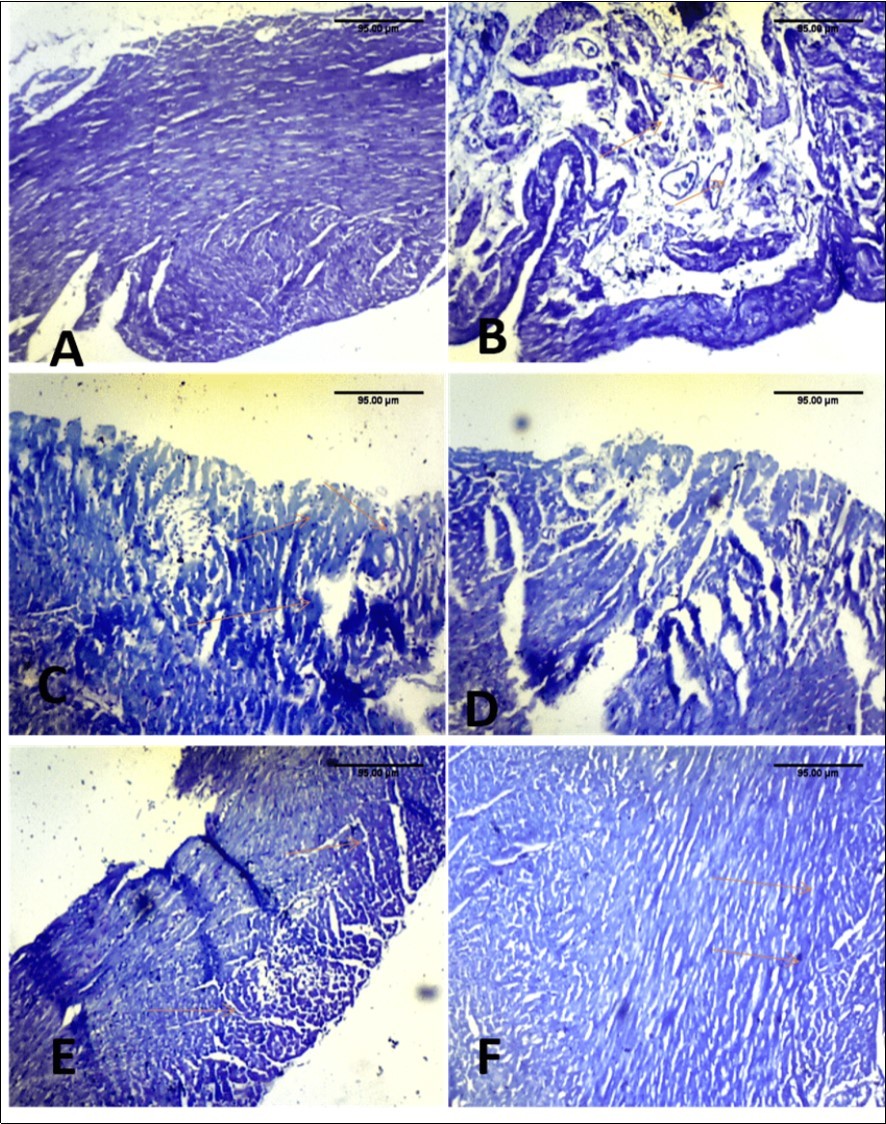
There was no significant difference in serum nitric oxide activity across all experimental groups (F=1.54, p = 0.22). However, the mean nitric oxide activity was lowest in group B (0.6476 ± 0.0074) and highest in the control group and group C (0.8948 ±0.1452) while groups D, E, and F had values of 0.8634±0.00363, 0.7938 ± 0.0074, 0.9006 ± 0.0374 respectively (Figure 5).
Figure 5.Effect of MEAS on serum nitric oxide activity of paraquat-exposed rat. n = 5, values are expressed as Nitric oxide (µ /L) ± SEM ( p = 0.22). (Group A- Normal Saline, Group B- Paraquat only, Group C-Paraquat + 100mg/kg MEAS, Group D- Paraquat + 200mg/kg MEAS, Group E- Paraquat + 100mg/kg Vitamin E, Group F – Paraquat only (7mg/kg) – Recovery)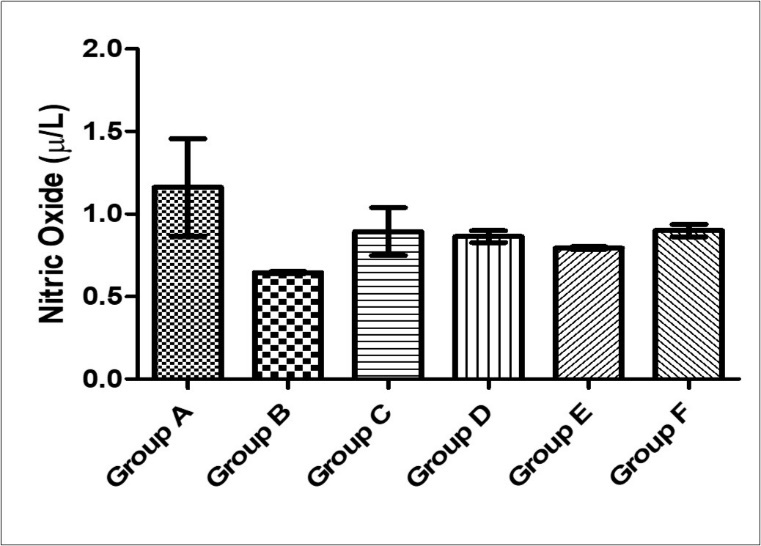
There was significant difference (F = 8.43; p = 0.0001) in Cytokeratin-18 activity across the experimental groups. Cytokeratin-18 activity in group B (903.1 ± 0.7071) was statistically higher than the control group (553.0 ± 6.837) while groups C, D, E and F (589.7 ± 86.78, 591.9 ± 78.47, 553.5 ± 0.94 and 734.6 ± 7.071) was significantly lower than group B also it was statically higher in F while compared with A, because group F was left untreated (Figure 6)
Figure 6.Effect of MEAS on glutathione reductase activity of paraquat-exposed rat. n = 5, values are expressed as glutathione reductase (mµ /ml) ± SEM (p = 0.08). (Group A- Normal Saline, Group B- Paraquat only, Group C-Paraquat + 100mg/kg MEAS, Group D- Paraquat + 200mg/kg MEAS, Group E- Paraquat + 100mg/kg Vitamin E, Group F – Paraquat only (7mg/kg) – Recovery).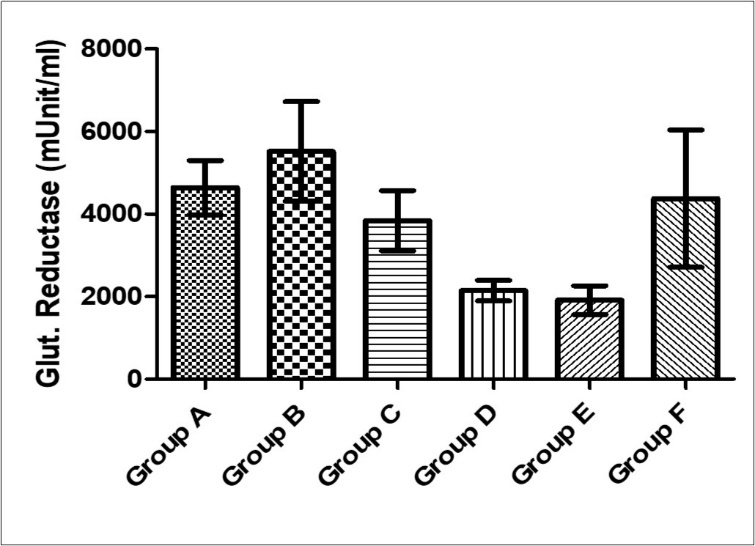
There was no significant difference in cardiac troponin I levels across the experimental groups (F= 1.49, p = 0.23). The cardiac troponin I activity was highest in group F (42.09 ± 13.16), while group B, C, D and E had values of 26.21 ± 0.113, 25.85 ± 0.17, 25.77 ± 0.04 and 26.51 ± 0.29 respectively (Figure 7).
Figure 7.Effect of MEAS on Cardiac Troponin I activity of paraquat-exposed rat. n = 5, values are expressed as cardiac Troponin I (pg/ ml) ± SEM( p = 0.23).(Group A- Normal Saline, Group B- Paraquat only, Group C-Paraquat + 100mg/kg MEAS, Group D- Paraquat + 200mg/kg MEAS, Group E- Paraquat + 100mg/kg Vitamin E, Group F – Paraquat only (7mg/kg) – Recovery).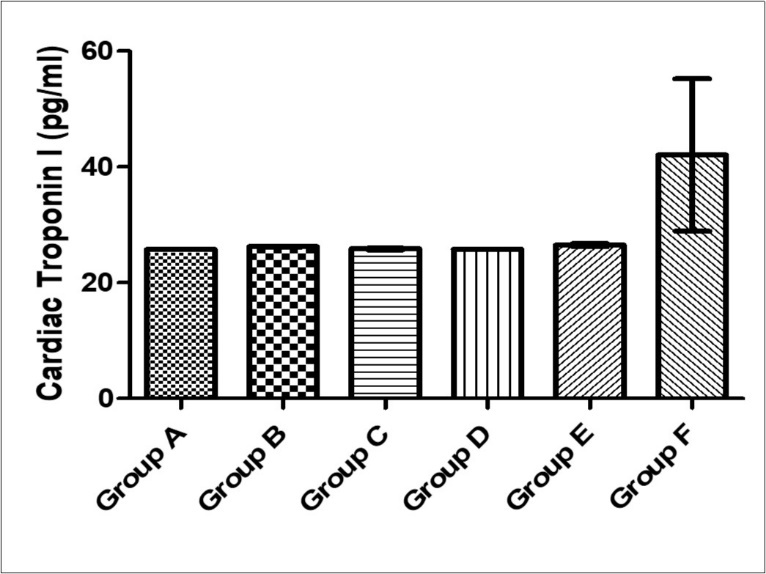
There was no significant difference in serum glutathione reductase across all experimental groups (F= 2.29, p = 0.08). Glutathione reductase activity was highest in group B (5519 ± 1207) and lowest in group E (1914 ± 349). Groups C, D and E had a mean value of 3841±730, 2148 ± 250 and 4374 ± 1662 respectively (Figure 8).
Figure 8.Effect of MEAS on Cytokeratin-18 activity of paraquat-exposed rat. n = 5, values are expressed as cytokeratin-18 (pg/ ml) ± SEM( p = 0.0001).(Group A- Normal Saline, Group B- Paraquat only, Group C-Paraquat + 100mg/kg MEAS, Group D- Paraquat + 200mg/kg MEAS, Group E- Paraquat + 100mg/kg Vitamin E, Group F – Paraquat only (7mg/kg) – Recovery).
Discussion
Phytochemical screening of methanolic extract of Abelmoscus esculentus seeds in this study revealed the presence of Tannins, glycosides, resins, saponins, flavonoids, sterols, phenols, carbohydrates and alkaloids, while terpenoids and phlobatannins were absent this is similar to findings, that analysed both aqueous extract (AE) and methanolic extract (ME) of Abelmoschus esculentus seeds and found the presence of alkaloids, carbohydrates, flavonoids, phenols, proteins, terpenoids, tannins, and sterols 22. Saponins and cardiac glycosides were found to be absent in AE and ME. Flavonoids have also been found to be the most potent antioxidative compound of plant phenolics 22.
The effect of paraquat exposure on the heart was investigated in this study alongside the cardio-protective effect of MEAS. In this study, the percentage weight change was lowest in group B (6.67%) rats and this was found to be reversed in the group treated with Vitamin E (19.28%) as well as MEAS, though the difference was not statistically significant. This could possibly be explained by the reduced food intake noted in this study similar to findings 23, who reported rapid and significant weight loss in up to 30% of paraquat-treated animals. According to findings, that assessed the weight of rats during and after paraquat treatment 8, no significant changes were observed in weight of paraquat treated group when compared to the saline treated control group. Also, no significant changes were observed after vitamin E treatment when compared to the saline treated control group which is in keeping with the results of this study. Abelmoschus esculentus may be beneficial in weight loss due to its cholesterol lowering effect which may explain why the Vitamin E treated group had a higher weight change than the MEAS treated group 24, though the percentage weight loss was not statistically significant. The lower feed consumption could also be due to direct adverse effect of paraquat on gastrointestinal tract, which is corrosive and strong irritant to the gastric mucosa and may lead to gastric ulceration and gastritis or peritoneal irritation due to the route of administration 25.
Oxidative stress is a condition which arises when the production of free radicals exceeds the body’s ability to neutralize and eliminate them. In healthy body, ROS and antioxidants remain in balance. When the balance is disrupted towards an over-abundance of ROS, oxidative stress occurs 26. An increase in antioxidant activity has been reported in mice exposed to particulate air pollution or cigarette smoke 27, 28.
Nitric oxide is an important signaling molecule that reacts with superoxide derived from the paraquat redox cycle, to form the potent oxidant peroxynitrite, which causes serious cell damage. The level of nitric oxide in this study was reduced in the paraquat-exposed groups and this is similar to findings that reported reduced NO bioavailability in the vessel wall in excess ROS generation 29. This could be due to oxidative inactivation of NO by the excessive generation of ROS like superoxide in the heart. This finding may suggest that high amount of oxidative stress can cause exhaustion of circulating nitric oxide and that paraquat may have a direct enzymatic denaturation on endothelial/inducible nitric oxide synthase.
Cytokeratin 18 (CK18) is a 45 kDa acidic intermediate filament protein. It is normally co-expressed with cytokeratin 8 and is found in most simple ductal and glandular epithelia. In this study, group B rats were found to have increased level of cytokeratin 18 which is suggestive of myocardial ischaemia similar to findings that reported increased levels of IgA anti-CK18 antibodies in ischemic heart disease pointer 30.
Troponin I is the biomarker of choice for the detection of cardiac injury. In this study, the level of cTnI was highest in group F rats which showed marked elevation and may be a pointer to chronic myocardial injury similar to reports that circulating troponin levels reflect chronic sources of myocardial injury and predict long term heart failure risk 32.Poisoning may lead to toxic myocarditis or dysfunction of the sinus node 33.
Glutathione reductase catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH), which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell. Activity of glutathione reductase (GR) markedly increased with sub lethal concentrations of paraquat in Maize strains 34. This was similar to findings in this study where untreated paraquat-exposed groups exhibited increased glutathione reductase (GR) while the level of GR was reduced in the treated groups. This suggests the presence of ROS and the ameliorative effects of MEAS; it also suggests that oxidative stress could stimulate glutathione reductase synthesis as a protective response.
Neutrophils can be identified by their granular cytoplasm and their multilobular, condensed nuclei. Because of their nuclear morphology, they are frequently also called “polymorphonuclear leukocytes” (aka "PMNs" or “polys”). Neutrophils generally enter tissues in large numbers only in response to a disease stimulus. In this study, acute exposure to paraquat was found to cause distortion of normal histo-architecture of the heart with tissue loss and marked neutrophil infilteration similar to findings that reported myocardial injury in severe paraquat poisoning which may explain the neutrophil infiltration found in this study 35, 36. In this study, there was presence of mast cell in acute paraquat toxicity which maybe a pointer to undergoing hypersensitivity reaction in paraquat toxicity.
Conclusion
The findings in this study suggest that paraquat exposure induced varying degrees of myocarditis as evidenced by marked neutrophil infiltration of tissue and myocyte loss. The study also concluded that during acute paraquat toxicity, mast cells are deposited in the heart tissue, as well as a change in the collagen-elastic fibre deposit in paraquat exposed rats. These changes were reversed by MEAS and Vitamin E. It also indicates that paraquat could trigger adaptive responses that counterbalance the potentially damaging activity of oxygen radicals and limiting further oxidant-mediated inflammation. Finally, it was found that MEAS and Vitamin E had comparable antioxidant effect and was able to reverse some of the responses initiated by acute paraquat toxicity.
References
- 1.A M Zain. (2007) The evaluation of the toxic effect of paraquat and its mechanism of action on reproductive system of male rats: Thesis. Universiti Sains Malaysia, Penang. http://eprints. usm.;.
- 2.Gil H, Hong J, Jang S. (2014) Diagnostic and therapeutic approach for acute paraquat intoxication’,Journal of Korean medical. 5(4), 124-125.
- 4.T J Haley.Review of the Toxicology of Paraquat (1, 1′-Dimethyl-4, 4′-bipyridinium Chloride)’.ClinicalToxicology1979;14:. 1-46.
- 5.Lee H-L, Lin H-J, Yeh S T Y, Chi C-H, Guo H-R.Presentations of patients of poisoning and predictors of poisoning-related fatality: findings from a hospital-based prospective study’,BMC public health2008;. 8, 7.
- 6.A C Cristóvão, Choi D-H, Baltazar G, M F Beal, Kim Y-S.. The Role of NADPH Oxidase 1–Derived Reactive Oxygen Species in Paraquat-Mediated Dopaminergic Cell Death’,Antioxidants & RedoxSignaling2009; 11, 2105-2118.
- 7.Wang S, Guo W, Ren J.Stress Signaling in Paraquat-Induced Target Organ Toxicity’,Reactive Oxygen Species2016; 1 :. 131-140.
- 8.M A Fahim, F C Howarth, Nemmar A, M A Qureshi, Shafiullah M et al. (2013) . Vitamin E Ameliorates the Decremental Effect of Paraquat on Cardiomyocyte Contractility in Rats, PLoSONE. Edited by Chen. X. 8, 3.
- 9.Wang s, Guo W, Ren J. (2016) Stress Signaling in Paraquat-Induced Target Organ Toxicity. Reactive Oxygen Species. 1(2), 131-140.
- 10.Lv D, Cheng X, Tang L, Jiang M. (2017) The cardioprotective effect of total flavonoids on Myocardialischemia/reperfusion in rats. Biomed Pharmacother. 88, 277-284.
- 11.H Van Remmen, Qi W, Sabia M, Freeman G. (2004) Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress’,Free Radical Biology. 36(12), 1625-1634.
- 12.Blaustein A, Schine L, Brooks W. (1986) Influence of exogenously generated oxidant species on myocardial function. Am j physiol. 250(4), 595-9.
- 13.Ren D, Chen G. (2010) Inhibition effect of okra polysaccharides on proliferation of human cancer cell lines’,Food Sci. 31, 353-356.
- 14.M A Ebrahimzadeh, S F Nabavi, S M Nabavi.and Eslami, B.‘Antihypoxic and antioxidant activity of Hibiscus esculentus seeds’,Grasasyaceites2010;. 61, 30-36.
- 15.Joseph J, Shukitt-Hale B, N A Denisova, Martin A, Perry G et al. (1999) Copernicus revisited: amyloid beta in Alzheimer’s disease.’,Neurobiology of aging. , Boca Raton 22, 131-46.
- 16.C A Murray, M A Lynch. (1998) Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus.’,The Journal of biological chemistry. , American Society for Biochemistry and Molecular Biology 273, 12161-8.
- 17.Zandi P P, Anthony J C, Khachaturian A S, Stone S V, Gustafson D et al. (2004) Reduced Risk of Alzheimer Disease in Users of Antioxidant Vitamin Supplements,Archives of Neurology. , American Medical Association 61(1), 82-88.
- 18.Ayasolla K, Khan M, A K Singh, Singh I.Inflammatory mediator and β-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E’,Free. , Radical Biology & Medicine2004; 37, 325-338.
- 19.N M Ansari, Houlihan L, Hussain B, Pieroni A. (2005) Antioxidant activity of five vegetables traditionally consumed by South-Asian migrants in. , Bradford, Yorkshire, UK, Phytotherapy Research 19, 907-911.
- 20.Imafidon C E, Akomolafe R O, Abubakar S A, Ogundipe O J. (2015) Amelioration of cadmium induced nephropathy using polyphenol-rich extract of Vernonia amygdalina (Del.) leaves in rat model. Macedonian journal of medical sciences. 3(4), 567.
- 21.F O Abulude, Akajagbor C, B H Dafiewhare.Advances in food sciences AFS. official journal of the Mediterranean Scientific Association of Environmental Protection (MESAEP) and the International Academy of Environmental Safety (IAES)., Advances in food sciences.ParlarScientificPubl2010; 20, 40-45.
- 22.S K Doreddula, S R Bonam, D P Gaddam, Desu B S R, Ramarao N et al. (2014) Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.). in Mice’,Scientific WorldJournal,Article ID 519848-14.
- 23.Dinis-Oliveira R, Sousa C, Remiao F, Duarte J.Full survival of paraquat-exposed rats after treatment with sodium salicylate’,Free Radical Biology2007;. 42(7), 1017-28.
- 24.N D Zaharuddin, M I Noordin, Kadivar A. (2014) The use of Hibiscus esculentus (Okra) gum in sustaining the release of propranolol hydrochloride in a solid oral dosage form.’,BioMed research international. Hindawi Publishing Corporation. 735891.
- 25.Debe E B, Okolonkwo B N, Ngokere A A. (2007) Toxicological effects of paraquat on the histology of the stomach, small intestine and testis of male albino rat (<i>Rattus norvegicus</i>)’,Port Harcourt Medical Journal. College of Health Sciences. , University of Port Harcourt 2, 51-55.
- 26.Agarwal A, Gupta S, R K Sharma, Morris S, Mugusi F et al. (2005) S.‘Role of oxidative stress in female reproduction’,Reproductive Biology and Endocrinology. BioMed Central. 3, 28.
- 27.Valenca S S, Silva B F, Lopes A A, Romana-Souza B, Marinho M C et al. (2008) Oxidative stress in mouse plasma and lungs induced by cigarette smoke and lipopolysaccharide’. , Environmental Research 108, 199-204.
- 28.Nemmar A, Al-Salam S, Dhanasekaran S, Sudhadevi M, Ali B.H.‘Pulmonary exposure to diesel exhaust particles promotes cerebral microvessel thrombosis: Protective effect of a cysteine prodrug l-2-oxothiazolidine-4-carboxylic acid’,Toxicology2009;. 263-84.
- 29.Kojda G, Harrison D. (1999) Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure’,Cardiovascular research. 43(3), 652-671.
- 30.Jahn L, Kreuzer J, E von Hodenberg, Kübler W, Franke W W et al.Cytokeratins 8 and 18 in smooth muscle cells. Detection in human coronary artery, peripheral vascular, and vein graft disease and in transplantation—associated arteriosclerosis.’.Arteriosclerosis, Thrombosis, and Vascular Biology1993;. 13, 11.
- 31.Lemos J A de, Braunwald E.ST segment resolution as a tool for assessing the efficacy of reperfusion therapy’,Journal of the American College of. Cardiology2001;38: 5.
- 32.Song C, Kan B, Yu G, Jian X, Wang J et al. (2014) Experimental and therapeutic medicine., Experimental and Therapeutic Medicine. , [Spandidos Pub.] 8(5), 1459-1462.
- 33.Hagen T, Brown L, Jones D.Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells’.BiochemicalPharmacology1986;. 35, 4537-4542.
- 34.Kang C, S C Kim, S H Lee, J H, D S Kim et al. Public Library of Science (2013) Absolute Lymphocyte Count as a Predictor of Mortality. in Emergency Department Patients with Paraquat Poisoning’,PLoSONE. Edited by 8, 78160.
