Abstract
Novel cancer therapeutics are superior and prevalent in the current scenario although a subset may not be satisfactorily alleviated or undergo disease relapse with the adoption of conventional chemotherapeutic agents. Cancer cells can comfortably elude immune destruction as interaction of cancer cells with native immune cells within tissue microenvironment is a cogent factor in evasion of cancer cells from pertinent immune surveillance. Thus, cancer immunotherapy can be safely contemplated as an efficacious and contemporary treatment modality for managing various malignant disorders.
Author Contributions
Academic Editor: Pietro Scicchitano, Cardiology Department, Hospital of Ostuni (BR) - Italy.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Anubha Bajaj
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Preface Immune system is a critical contributor in determining the outcomes of diverse, incipient malignancies. Immune system can perform as a tumour promoter by augmenting tumour evolution, facilitating cellular metamorphoses and modifying the immunogenicity of malignant cells. Tumour suppression is also undertaken by extrinsic mechanisms such as decimation of emerging tumour or restriction of tumour expansion. Nevertheless, clinically detectable tumefaction can ensue in immunocompetent individuals, partially on account of tumour induced immune suppression.
Novel cancer therapeutics are superior and prevalent in the current scenario although a subset may not be satisfactorily alleviated or undergo disease relapse with the adoption of conventional chemotherapeutic agents. Cancer cells can comfortably elude immune destruction as interaction of cancer cells with native immune cells within tissue microenvironment is a cogent factor in evasion of cancer cells from pertinent immune surveillance. Thus, cancer immunotherapy can be safely contemplated as an efficacious and contemporary treatment modality for managing various malignant disorders.
Principle and Premise Immune checkpoint therapy is devised on the premise that immune checkpoint molecules appear to function and prevent autoimmune manifestations or specific tissue deterioration in the course of accrued pathogenic infection. Checkpoint molecules are essentially constituted of inhibitory receptors which are elucidated upon surface of T lymphocytes and tumour cells and are intermediary to functional interrelation betwixt the cellular varieties 1, 2.
Immune suppression is mediated by specific immunomodulatory receptors such as cytotoxic T- lymphocyte associated antigen- 4 (CTLA-4) and programmed death ligand-1(PD-1), principally enunciated upon T lymphocytes. Therapies contingent to monoclonal (mAb) antibody, which specifically target CTLA-4 and /or PD-1, are cogitated as checkpoint blockade and are beneficial with consistent therapeutic responses in diverse malignancies 3, 4.
Adaptive immune resistance is designated on account of interplay of immune checkpoint molecules upon T lymphocytes and cancer cells. The manoeuver restricts cytotoxic capacity of T lymphocytes and enables tumour cells to evade the impact of cytotoxicity.
Extrinsic immune inhibition incurred with T lymphocytes can induce the secretion of inhibitory molecules such as transforming growth factor β(TGF-β), interleukin 10 (IL-10) and indoleamine 2,3 dioxygenase (IDO). Aforesaid measures reduce the functional capacity of cytotoxic T lymphocytes with a subsequent decline of recruitment of anti- inflammatory cells, regulatory T lymphocytes (T reg) and myeloid derived suppressor cells (MDSC) 1, 2.
Malignant cancers are discriminated into distinctive categories as
1) Immunologically ignorant cancers which demonstrate minimal mutations, are immune tolerant towards self- antigens and demonstrate a lack of infiltrating T lymphocytes.
2) Immunologically responsive cancers delineate a multitude of infiltrating T lymphocytes which indicate T lymphocyte initiated intrinsic immune inhibition in addition to T lymphocyte procured extrinsic, tumour concordant immune suppression.
Immune inhibition engendered with T lymphocytes is commonly mediated through activation of immune checkpoint molecules such as cytotoxic T lymphocyte associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), T cell immunoglobulin mucin -3 (Tim-3) and lymphocyte –activation gene 3 (LAG-3) 1.
T cell immuno-receptor with immunoglobulin and ITIM domain (TIGIT) is an immune checkpoint molecule with inhibitory activity which is enunciated upon cogent immune cells especially regulatory T cells (T regs) and natural killer (NK) cells. Enhanced TIGIT/CD226 ratio of expression can appear upon regulatory T cells in association with decline in cytokine production and consequently an inferior survival 2, 3.
Applicable biomarkers in CTLA-4 checkpoint therapy Cytotoxic T lymphocyte associated antigen 4 (CTLA-4) is solely enunciated upon T lymphocytes. CTLA-4 regulates the activation of T lymphocytes and prohibits function of CD28 immune molecule. Blockade of CTLA-4 ensures a helper T lymphocyte dependent augmentation of suppressive function of regulatory T cells.
Tumour infiltrated lymphocytes (TIL) are a feature considered as an indicator of favourable clinical outcome. Emergence of lymphocytes within tumour tissue is a superior biomarker of immune- therapeutic blockade.
Absolute lymphocyte count generates a heterogeneous population of lymphocytes. Anti CTLA-4 therapy is not concurrent to absolute lymphocyte count in predicting clinical outcomes. Malignant tumours depicting an absolute neutrophil count exceeding > 7500 neutrophils delineate a decreased overall survival (OS) and progression free survival (PFS). An enhanced neutrophil to lymphocyte ratio (NLR) preceding administration of anti CTLA-4 therapy is accompanied with an inferior prognosis. Inducible Co-Stimulator (ICOS) is exemplified upon the cellular surface of activated T lymphocytes and is incriminated in multiplication and survival of T lymphocytes. Carcinoma bladder, carcinoma breast and mesothelioma subjected to anti CTLA-4 therapy can demonstrate an amplification of CD4+ ICOS+ T lymphocytes, a feature which can be considered as a pertinent biomarker for monitoring clinical outcomes of various malignancies 3, 4.
T cell Receptor (TCR): Human T cell receptor preserves autoimmune functions and limits the pathogenicity of various infective or malignant, incriminating factors. Enunciation of diverse T cell receptors usually delineate a superior therapeutic response rate. Enhanced variability of T cell receptors is concomitant to reduced overall survival, in contrast to delineation of stable phenotype of T cell receptors. However, anti CTLA-4 therapy accompanied by augmentation of CD8+ immune response may not be associated with an ameliorated clinical outcome.
Tumour associated antigens (TAA): Cancer cells usually exemplify a variety of tumour associated antigens. Administration of anti CTLA-4 therapy amplifies TAA specific antibodies. Enhanced production of antigen specific CD4+ and CD8+ T lymphocytes are also delineated. Aforesaid cellular reaction is cogitated in malignant melanoma, carcinoma ovary and carcinoma prostate 4, 5.
Certain tumours such as malignant melanoma display an intense reactivity to cancer- testis antigen NY-ESO-1. Malignant melanoma treated with anti CTLA-4 therapy and immune reactive to NY-ESO-1 depicts superior clinical results, in contrast to tumours non immune reactive to NY-ESO-1.
Melan A is a significantly enunciated biomarker associated with anti CTLA-4 therapy, which is enhanced in malignant melanoma and carcinoma prostate with demonstrably favourable clinical response and overall survival 4, 5.
Myeloid Derived Suppressor Cells (MDSC) Cogent cellular compartment is comprised of a heterogeneous population of precursor and progenitor myeloid cells which can effectively serve as antigen presenting cells. The particular cells can be observed in various solid tissue malignancies such as carcinoma breast or head and neck and squamous cell carcinoma, non small cell lung cancer (NSCLC) and carcinoma pancreas. MDSC configures molecules which can function effectively in immune suppression and are comprised of arginase 1, interleukin 10 (IL-10) and transforming growth factor- β(TGF-β). Elevation of MDSC depicts augmented disease activity in malignant melanoma. Declining frequency of MDSC is associated with enhanced survival in individuals with malignancies.
Regulatory T cells (T reg) elucidate an expansion within the peripheral blood of subjects inflicted with malignancy. T lymphocytes can enunciate FOXP3 which is a surrogate biomarker for regulatory T lymphocytes and is associated with favourable clinical outcomes, particularly in carcinoma breast and colorectal carcinoma5, 6.
Cancer immunotherapy can depict an accumulation of MDSC and regulatory T cells along with a component of CD4+FOXP3+CD25hi cells. Soluble CD25 can capture interleukin-2 (T cell activation) and reduce the efficiency of anti CTLA-4 antibody. Amalgamated CD4+FOXP3+CD24hi cells can induce anti-tumour immune suppression and result in an inferior clinical prognosis.
Indoleamine 2,3- dioxygenase (IDO) functions as an enzyme which renders incapable tryptophan, a process which contributes to immune suppression within the tumour microenvironment. Subsequently, suppression of T lymphocytes is noted besides an amplified activation and engagement of regulatory T cells and MDSC, a process which further reduces T cell activity in response to tumour cells3, 4.
Enunciation of microbiomes : Cancer cells when treated with anti PD-1 agents can enhance the activity of dendritic cells. Administration of antibiotics can compromise anti-tumour influences of anti PD-1 therapy. Subjects demonstrating an unfavourable segment of bacteria colonizing gastrointestinal tract such as the Bacteriodales species can elucidate a defective systemic and anti- tumour immune response. In contrast, colonization of commensal microbiomes display an enhanced efficacy of anti PD-1 therapy1, 2.
Biomarkers for PD-1/PD-L1 Checkpoint Therapy PD-1 molecule is active in prohibiting the activity of T lymphocytes in pro- inflammatory conditions and restricting autoimmunity. PD-1 receptors emerging within T lymphocytes are activated and adhere to associated ligands PD-L1 and PD-L2 . Consequently, the configured immune check point inhibits T cell function. Thus, PD-1, PD-L1 bio-axis controls T cell activation, prevents damage to adjacent soft tissue in pro-inflammatory conditions and engenders a mechanism by which malignant cells evade immune surveillance within the tumour microenvironment. A surrogate biomarker for tumours subjected to anti PD-1 therapy is cogitated with PD-L1. Enhanced enunciation of PD-L1 within malignancies is concordant to immune evasion and demonstrates an inferior prognosis in subjects administered cancer immune therapy. Exemplification of PD-L1 can be influenced by tumour infiltration with T lymphocytes which generate interferon ɣ (IFN-ɣ), a process which depicts a superior clinical outcome. Malignancies demonstrating amplified enunciation of PD-L1 usually exhibit an enhanced response rate, progression free survival and overall survival 5, 6.
JAK kinases concurrently appear in downstream signalling with the delineation of interferon ɣ (IFN ɣ). Whole exome sequencing of subjects with initially efficacious and subsequent refractoriness to anti PD-1 therapy can demonstrate chromosomal mutations of JAK1/JAK2 signalling pathway. Loss of function mutations inhibit anti- tumour activity with a consequent activation of T lymphocytes which can attack cancer cells. Thus, JAK/ STAT signalling pathway can mediate in order to engender evasion of cancer cells from influence of immune checkpoint blockade 6, 7.
Mutational Egress Cancer cells exhibit innumerable somatic mutations. Tumours delineating significant mutations can augment neo antigen –specific CD4+ and CD8+ T lymphocytes. Additionally, PD-1/PD-L1 checkpoint blockade increases endogenous immunity in concordance with neo antigen- specific CD4+ and CD8+ immune reactive T lymphocytes demonstrating chromosomal mutations. Mismatch repair deficiency (MMRD) and microsatellite instability (MSI) can be utilized as biomarkers for predicting malignancies with superior clinical outcome. Prospective mechanics and modifications in enhanced quantification of genomic mutations unresolved with DNA mismatch repair tend to amplify the immunogenicity of tumour cells. Carcinomas deficient in mismatch repair demonstrate an amplified infiltration of cytotoxic T lymphocytes, thus indicating a robust immune response to the tumour 1, 2. Figure 1, Figure 2, Figure 3, Figure 4, Figure 5.
Figure 1.Various components of active and passive modalities of cancer immunotherapy 12.
Figure 2.Methodolgies of anti CTLA-4 and anti PD-1 therapy in cancer immunotherapy 13.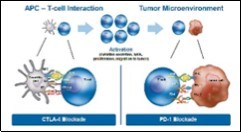
Figure 3.Effect of various immune cells upon target cancer cell in cancer immunotherapy 14.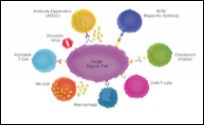
Figure 4.Functions of T cell receptor (TCR) in cancer immunotherapy 15.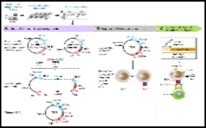
Figure 5.Contribution of various cytokines in cancer immunotherapy 16.
Histocompatibility Antigens Genotype of human leukocyte antigen (HLA- 1) contributes to immune response to cancer. Efficacy of administered anti CTLA-4 and anti PD-1 therapy is contingent to the immune activity specific for HLA -1 molecules. Heterozygous HLA-1 loci demonstrate an enhanced survival, in contrast to homozygosity and minimal mutations within singular or multiple genetic loci of HLA- 1. Also, homozygosity of HLA –B and loss of heterozygosity (LOH) at HLA-1 are accompanied by a declining overall survival. Homozygosity at HLA-1 genetic loci or loss of heterozygosity (LOH) at HLA-1 loci can raise genetic barricades to the employment of cancer immunotherapy 1, 2.
However, cogent tumour antigens emerging as a target of T lymphocytes and mobilized by checkpoint blockade immunotherapy remain obscure. Employment of aforesaid tumour antigens for engendering pertinent, tumour-specific cancer vaccines is a feature necessitating exploration 3, 4.
Adoption of Neo-antigens Endogenous mutations of cancer proteins are cogitated as neo-antigens which are usually represented upon the extraneous surface of malignant cells. Neo-antigens pro-actively segregate immune cells from tumour cells and can be contemplated as targets for immunotherapy. Neo-antigens are commonly discerned in cholangiocarcinoma, leukaemia, malignant melanoma, non small cell lung cancer (NSCLC) and carcinoma ovary. Administered anti CTLA-4 and anti PD-1 therapy, in association with genomic mutations and enhanced neo- antigens, is concurrent with clinical outcomes 1, 2.
Natural Killer Cells Anti PD-1 immune-therapy can prove to efficacious in certain instances whereas specific cancers can be refractory to the modality. Natural killer (NK) cells are cytotoxic T lymphocytes which can mediate an immune response through secretion of chemokines and cytokines. Enhanced quantification of natural killer cells is indicative of superior prognosis, particularly in solid tumours such as metastatic carcinoma prostate, colorectal carcinoma or malignant melanoma. Natural killer cells tend to mobilize dendritic reticulum cells within the tumour microenvironment. Type 1 dendritic reticulum cells (cDC1) are known to propagate anti- tumour activity through a process of T lymphocyte recruitment and secretion of interleukin 2 (IL2), with a consequently elevated production of tumour infiltrating lymphocytes (TILs). Declining levels of type 1 dendritic reticulum cells (cDC1) are usually associated with inferior prognosis in subjects of cancer immunotherapy1, 2.
Proliferative Markers Ki-67 is contemplated as a surrogate biomarker for assessing proliferation of T lymphocytes. Regulatory T lymphocytes demonstrate enhanced Ki-67 values. Prospective cytotoxicity of CD8+ T lymphocytes are enunciated with reactive Ki67+ and PD-1+ biomarkers and a reactive granzyme B. Additionally, minimal Bcl-2, an elevated ICOS and reactive costimulatory molecules CD27+ and CD28+ can be exemplified. An amplified Ki- 67 reaction upon CD8+ T lymphocytes, associated with anti PD-1 therapy, is concurrent with a superior therapeutic outcome.
Tumour Immune Dysfunction and Exclusion (TIDE) is designated as a computational modality which demonstrates primary methodologies of immune evasion by cancer cells. Dysfunctional T lymphocytes occur within tumours with enhanced cytotoxic T lymphocytes besides an impaired infiltration of T lymphocytes emerging within cancers with decimated values of cytotoxic T lymphocytes. Thus, amplification of T lymphocyte dysfunction is associated with unresponsiveness to anti PD-1 therapy or anti CTL-4 therapy [1,2).
Chimeric Antigen Receptor (CAR) T cell immunotherapy can be adopted to treat several malignancies. CAR T cells are genetically engineered, autologous T lymphocytes which enunciate chimeric antigen receptors against B-lineage antigen CD19. CD19 is exemplified upon cancer cells and CAR T cell therapy can be cogently applied to manage diffuse large B cell lymphoma (DLBCL) and B- cell precursor acute lymphoblastic leukaemia (B-ALL). Remission proportions of 60% to 90% are exemplified in adult and paediatric subjects of refractory and relapsed B- cell precursor acute lymphoblastic leukaemia ((B- ALL) 1, 2.
Therapeutic Indications Contemporary cellular cancer immunotherapies are designated as the approved molecules of tisagenlecleucel and axicabtagen-ciloleucel and can be employed for treating diffuse large B cell lymphoma and acute lymphoblastic leukaemia7, 8.
Genetically engineered anti CD19 chimeric antibody receptor (CAR T) cells are obtained with leucapheresis. Lympho- depleting chemotherapy is employed with subsequent, in vivo augmentation of anti CD19 CAR T cells. CAR T cells can be contemplated as a cogent therapy for managing solid malignancies such as chronic lymphocytic leukaemia, multiple myeloma and gastrointestinal cancers. CAR T cell therapy can be adopted in combination with variant strategies to enhance therapeutic safety such as
modifications of the chimeric antigen receptor cell
Recognition of biomarkers indicative of CAR T cell toxicity
Employment of safety switches such as “inducible suicide genes”
Novel therapeutic agents to alleviate toxicity of CAR T cells such as cytokine release syndrome (CRS) and neurologic events (NE)(7,8).
Genetic modifications of T cell receptor (TCR) or adoptive transposition of indigenous, natural tumour reactive T lymphocytes or tumour infiltrating lymphocytes (TIL) which can be retrieved from autologous tumour tissue or tumour draining lymph nodes can be achieved. Engendering lymphocytes with modified T cell receptor (TCR) is contingent to human leucocyte antigen (HLA) haplotype and can generate an unsuspected off-target toxicity. T cell receptor (TCR) with native or tumour reactive T lymphocytes can be beneficially employed in treating malignant melanoma. Autologous tumour reactive T cells usually identify the cogent carcinoma in concordance with unaltered, native T cell receptor (TCR) 9, 10.
Reinfusion of tumour infiltrating lymphocytes (TIL) or tumour reactive T cells as collected from tumour tissue or tumour draining lymph nodes can be adopted following lympho-depleting chemotherapy and accompanying intravenous interleukin-2 (IL-2). Aforesaid methodology is considered beneficial in managing advanced malignant melanoma unresponsive to PD-1/PD-L1 blockade and can be successfully employed in metastatic gastrointestinal carcinoma, carcinoma breast, ovary and endometrium or urothelial carcinoma. Also, colorectal carcinoma and cholangiocarcinoma can depict appropriate results with the employment of cogent immunotherapy 10, 11.
Mutant, tumour-specific proteins appear as a significant component of antigenic immune rejection by T lymphocytes with the adoption of α PD-1 and/or α CTLA-4 therapy. Additionally, therapeutic application of synthetic long peptide (SLP) vaccines incorporating cogent mutant epitopes can initiate tumour rejection in concurrence to checkpoint blockade therapy. Mutant, tumour- antigen specific T lymphocytes can emerge in progressive malignancies, can be reactivated sequential to therapy with α PD-1 and /or α CTLA-4 and can demonstrate treatment-specific, transcriptional profiles which can induce tumour rejection. Diverse, tumour- specific, mutant antigens (TSMA) are precise targets of checkpoint blockade therapy and can be employed to manufacture personalized, cancer- specific vaccines and scrutinize the mechanics of various, pertinent checkpoint blockade therapies 10, 11. Table 1.
Table 1. Assessment of biomarkers in cancer immunotherapy 1.| Technologic Utilization | Therapeutic Mode |
| Whole exome sequencing | Mutational load for anti CTLA-4 and anti PD-1 therapy |
| Neo antigen identification with CD8+ T lymphocytes | |
| Gene expression technology | Intrinsic and extrinsic immunosuppressive molecules |
| Gene expression profiles | |
| Integrating outcomes of adjunctive analytical techniques | |
| Epi-genomics | Interaction of histone modifications and DNA methylation |
| Validation for assessing biomarkers in immunotherapy | |
| Proteomics | Chemokines, cytokines and soluble factors |
| Tumour associated antigens (TAA) and their antibodies | |
| Minimal sample volumes, enhanced sensitivity, specificity and data generation of high-dimensional data | |
| Flow Cytometry | Phenotype and function of immune cells with multiple probes |
| Fluorescence spectral overlap can restrict results | |
| Mass Cytometry | Simultaneously discerns multiple biomarkers than flow cytometry |
| Measuring immune cell phenotype | |
| Expensive, gradual collection with limited cell recovery | |
| B and T cell sequencing | Sensitive and reproducible quantification of B and T cells |
| Clonal TIL to assess response to anti PD-1 therapy | |
| Multiplexed immunohistochemistry | Sample phenotype, H-scoring, positive/negative response, density measurements, spatial pattern point analysis. |
| Role and function of regulatory T cells (Treg) in anti CTLA-4 therapy | |
| Density of CD8+ T cell infiltrates with anti PD-1 therapy | |
| Simultaneous detection of multiple biomarkers | |
| Expensive, sample size and time are restrictive | |
| Radiomics | Enhanced radiomic or CD8+ score is related to overall survival and improved treatment response |
References
- 1.George A P, Kuzel T M. (2019) The discovery of biomarkers in cancer immunotherapy”. , Comput Struct Biotechnol J 17, 484-497.
- 2.Kruger S, Ilmer M. (2019) Advances in cancer immunotherapy 2019-latest trends “. , Journal of Experimental and Clinical Cancer Research 38-268.
- 3.Tang J, Shalabi A. (2018) Comprehensive analysis of the clinical immuno-oncology landscape”. , Ann Oncol 29(1), 84-91.
- 4.Gubin M M, Zhang X. (2014) Checkpoint blockade cancer immunotherapy targets tumour-specific mutant agents”. , Nature 515(7528), 577-581.
- 5.Galluzi L, Buque A. (2015) Immunological effects of conventional chemotherapy and targeted anticancer agents”. , Cancer Cell 28(6), 690-714.
- 6.Huang A C, Postow M A. (2017) T-cell invigoration to tumour burden ratio associated with anti PD-1 response”. , Nature 545(7652), 60-5.
- 7.Ribas A. (2015) Adaptive immune resistance: how cancer protects from immune attack”. , Cancer Discov 5(9), 915-919.
- 8.Kelderman S, Schumacher T N. (2014) Acquired and intrinsic resistance in cancer immunotherapy”. , Mol Oncol 8(6), 1132-1139.
- 9.Shin D S, Ribas A. (2015) The evolution of checkpoint blockade as a cancer therapy : what’s here, what’s next?” Curr Opin Immunol. 33, 23-25.
- 10.Pardoll D M. (2012) The blockade of immune checkpoints in cancer immunotherapy”. , Nat Rev Cancer 12, 252-264.
