Abstract
Gold nanorods (GNRs) are plasmonic nanostructures by virtue of their size-dependent optical properties, offer a bionanotechnology platform in areas of bioimaging, drug delivery etc for disease diagnosis, prognosis, and therapy. GNRs are more sensitive to changes in local environments, and offer strong scattering and absorption efficiencies thus providing opportunities to integrate multiple imaging modes and therapeutic strategies. The hydrodynamic size of these GNR under physiological condition is <100 nm, making them ideal as intracellular delivery agents. RNA interference using small inhibitory RNA (siRNA) has become a powerful tool to downregulate mRNA levels by cellular nucleases that become activated when a sequence homology between the siRNA and a respective mRNA molecule is detected. siRNA is used to silence genes involved in the pathogenesis of various diseases and holds a promising option for the development of novel therapeutic strategies in neurological dysregulation such as that observed in drug addiction. However, a major challenge in gene therapy continues to be effective delivery of siRNA and its sustained release at targeted sites. Previously, we have shown the GNR coated with poly (diallyldimethyl ammoniumchloride) (GNR-PDDAC) electrostatically complexed to the dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) siRNA forming a GNR-nanoplex that was able to effectively silence the DARPP-32 gene expression in dopaminergic neuronal (DAN) cell cultures in- vitro. The current report, explores if modification of the surface coating properties of the GNRs with different surface coatings namely, amino terminated polyethylene glycol (GNR-PEG), polyethyleneimine (GNR-PEI) and Chitosan (GNR-CIT) alters their stability, cytotoxicity and DARPP-32 gene silencing efficiency in-vitro dopaminergic neuronal (DAN) cell cultures with the goal of determining the most suitable surface coating for the GNR that would provide a GNR-nanoplex with the most stability, least cytotoxicity and most efficacious gene silencing.
Author Contributions
Academic Editor: Tao Pang, Associate Professor, China Pharmaceutical University. Visiting Fellow, National Institute of Mental Health
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2014 Atcha Kopwitthaya, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Recent advances in the field of nanotechnology offer an unprecedented opportunity to enhance the power of siRNA mediated gene therapy by providing both an efficient delivery system as well as targeted specificity. Small interfering RNA (siRNA)-mediated knockdown of gene specific messenger RNA (mRNA) levels is a great therapeutic strategy 1, 2, 3, 4, 5, 6, 7, 8, in which, double-stranded RNAs are cleaved by the cellular nuclease Dicer into short 21–22mer fragments referred to as siRNA, which enter a ribonuclease protein complex termed the RNA-induced silencing complex. This complex mediates a specific degradation of the corresponding mRNA. Nanoparticles can form stable complexes with siRNAs (nanoplex: a complex between a nanoparticle and an siRNA) that can overcome all the impediments associated with siRNA in the free form. siRNA technology is a novel method to achieve complete and persistent knockdown of gene expression. Stable and sustained gene silencing is a key determinant as to whether or not RNAi-based therapeutics will have clinical relevance and gene delivery encompasses extracellular transport of the nanoplex to target cells, its intracellular RNA trafficking, and processing1, 2, 3, 4, 5, 6, 7, 8.
Gold nanoparticles are particularly attractive for therapeutic applications due to their biocompatibility and ease of complex formation with biomolecules. Gold-nanorods (GNR) have far reaching potential for the study of intracellular processes at the single-molecule level, using high-resolution cellular imaging, long-term observation of cell trafficking in vivo and gene silencing 9, 10, 11, 12, 13. The hydrodynamic size of these GNR-nanoplexes under physiological condition is <100 nm, making them ideal as intracellular delivery agents. Gold nanoparticles have been used for more than a decade as a gene carrier for plasmid DNA and oligonucleotides, but recently our group was the first to report the use GNR for the delivery of siRNA against DARPP-32 in vitro 14, 15, 16, 17, 18, 19, 20, 21. DARPP-32 plays a significant role in the activation of the dopaminergic signaling pathway which is related to addictive behaviors. DARPP-32 was initially discovered as a major target for dopamine-activated adenylyl cyclase and protein kinase A (PKA) in the striatum and is recognized to be critical to the pathogenesis of drug addiction. Drugs of abuse act on the dopaminergic system in the brain and perturb DARPP-32 function. Successful suppression of DARPP-32 gene expression may efficiently facilitate addiction therapy by inhibiting the drug abusing behavior. To silence DARPP-32 gene expression in dopaminergic neuronal cells, we used cationically charged PEGylated gold nanorods electrostatically coupled with negatively charged siRNA to form stable nanoplexes. We evaluated these nanoparticles as nonviral gene carriers by investigating their specificity and efficiency in achieving DARPP-32 gene silencing in primary dopaminergic neuronal cells, which are typically difficult to transfect. Gold nanoparticles (GNP) are an attractive drug delivery vector due to the ease with which biomolecules such as siRNA can be attached to the gold surface using thiol chemistry. This process can also allow attachment of multiple targeting or functional groups to the nanoparticle surface to produce a multifunctional nanoparticle 22, 23, 24, 25.
We and others have developed colloidal gold nanostructures as siRNA nanocarriers to improve cellular penetration, endosomal release, carrier unpacking and intracellular transport 14, 15, 16, 17, 18, 18, 20, 21, 22. However, if we want to extend our in vitro studies to in vivo or pre-clinical evaluations, issues such as stability, specificity and long term safety are still major concerns. For example, the major problems of naked siRNA delivery in vivo are rapid enzymatic degradation and substantial liver and renal clearance 19. The intrinsic cytotoxicity and high reactivity of the GNR in the physiological milieu have limited their applications in vivo 23. Our group has demonstrated that PEGylated gold nanorod formulations can be a promising nanocarrier in vivo because of their low toxicity and ability for prolonged systemic circulation up to 40 hours which allows nanoparticles to reach their targeted site 22, 23. It appears, that the surface coating of nanoparticles is the key factor that determines it stability, cytotoxicity and efficacy and therefore their utility for in vivo studies 24, 25. Therefore, the aim of this study was to develop a stable, nontoxic and cationic GNR based nanoparticle gene delivery system using four different types of modifications of the GNR surface coatings, which include, amino terminated polyethylene glycol (GNR-PEG), poly (diallyldimethyl ammoniumchloride) (GNR-PDDAC), polyethyleneimine (GNR-PEI) and Chitosan (GNR-CIT). These four separate cationic GNR with the above four different surface coatings were used as vehicles for DARPP-32 siRNA delivery in dopaminergic neuronal (DAN) cells. We did a systematic characterization of all four GNR formulations, investigated their ability to electrostatically bind to the DARPP-32 siRNA to form a nanoplex and evaluated their stability, cytotoxicity and gene silencing efficiency in an in-vitro dopaminergic neuronal cell culture system.
Materials and Methods:
Materials:
Gold (III) chloride trihydrate (HAuCl43H2O), 99.9+%, Sodium borohydride (NaBH4) 99%, hexadecyltrimethylammonium bromide (CTAB), ≥ 98%, L-Ascorbic acid, 99+%, Chitosan low molecular weight, Polyethyleneimine (PEI) and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma-Aldrich. Methoxyl poly(ethylene glycoside)-thiol (mPEG-SH, MW 2,000) and amino-poly(ethylene glycoside)-thiol (NH2-PEG-SH, MW 5,000) were purchased from Laysan Bio, Inc. Silver nitrate (AgNO3), 99.9+% was purchased from Alfa Aesar. Acetic acid was purchased from J.T. Baker. Poly(3,4-ethylenedioxythiophene)/poly(styrenesulfate) (PEDT/PSS, MW 240,000) and poly(diallyldimethyl ammonium chloride) (PDDAC, 20%) were purchased from Polysciences, Inc. All chemicals were used as received. HPLC grade water was used in all experiments. Stock solution of sodium borohydride, L-ascorbic acid, silver nitrate and hexadecyltrimethylammonium bromide (CTAB) were freshly prepared for each new set of experiments.
Synthesis of CTAB GNRs:
GNRs were obtained from method described in our previous work 9 Briefly, gold seed was prepared from 10 mL of 0.1 M CTAB solution, 200 µL of 25 mM HAuCl4, and 1 mL of ice-cold 0.01 M NaBH4 on vigorous stirring for 2 minutes. Then, growth solution was from mixing of 10 mL of 0.1 M CTAB, 200 µL of 25 mM, small amount of Ag+ ion (8 x 10-5 M) and 100 µL of 0.1 M ascorbic acid. Finally, the growth solution was heated up to be 33ºC in the water bath and 12 µL of seed solution was gently added to it. The rod formation was permitted undisturbed at 33ºC for at least 3 hours. Then, CTAB-coated Au NRs were centrifuged twice at 9000 rpm for 15 minutes. The pellet was redispersed in water for further use.
Preparation of Cationic Polymer Coated GNRs (GNR-PEI and GNR-PDDAC):
CTAB GNRs were changed to a negatively charged surface by coating with PEDT/PSS polyelectrolyte solution following a protocol from Ding et al 9. After an hour, on stirring, at room temperature, the negatively charge GNRs were centrifuged to remove excess PSS. Then, either cationic PEI or PDDAC were added to reverse the negative charge to positive (GNR-PEI and GNR-PDDAC respectively). After an hour of high speed stirring, cationic GNRs was centrifuged twice to remove unreact PEI or PDDAC, redispersed in HPLC water, and kept for further use.
Preparation of Chitosan Coated Gold Nanorods (GNR-CIT):
Chitosan capping GNRs were synthesized from a protocol described by Guo et al 26 with a slightly modification. Briefly, 25 mg of chitosan was dissolved in 5 mL of 1% acetic acid. Then, 17 mM of EDTA and 1 ml of as-prepared GNRs were mixed with chitosan for 5 hours. To form the colloidal nanoparticles, ethanol was added dropwise clear solution turned opalescent. Later, 30 μL of 25% Glutaraldehyde was introduced to cross link the chitosan nanoparticles at room temperature for 4 hours. Finally, chitosan coated GNRs were centrifuged twice at 6000 rpm for 30 minutes. The pallet was redispersed in HPLC water for further use.
Preparation of Amine Terminated PEG Gold Nanorods (GNR-PEG):
Similar to our PEGylated GNRs reported before 19, 2.5 mM mPEG-SH was stirred with CTAB GNRs in the presence of 0.5 mM NH2-PEG-SH for 24 hours. Amine terminated PEG GNRs, then, were centrifuged twice to remove excess PEG and redispersed in HPLC water for further use.
Nanoparticle Characterization:
UV-visible Absorption spectra were collected using an Agilent 8453 UV-visible spectrometer. The samples were measured against water as reference. Dynamic light scattering (Brookhaven 90PLUS with ZetaPALS option) was used to determine the size distribution. High-Resolution TEM images were obtained using a JEOL model JEM-2010 transmission electron microscope operating at an acceleration voltage of 200 kV. The specimens were prepared by dropping the sample onto an amorphous carbon-coated 300 mesh copper grid and allowing the solvent to evaporate.
Cell Culture:
Human dopaminergic neuronal precursor (DAN) cells were obtained from Clonexpress, Inc (Gaithersburg, MD;Cat No: DAN 020). Actively growing population of cells were tested for tyrosine hydroxylase (TH) expression by immunocytochemistry. DAN cells are supplied with a proprietary growth factor supplement (DNCS) as a 100X stock solution, which is added to DMEM:F12(50:50) containing 5% FBS and growth supplements to make DAN cell growth medium. The cells are grown in regular tissue culture dishes and subcultured at a split ratio of 1:2 at confluence. These cells differentiate into neurons within a week, when plated on polylysine (PLL) coated plates at a density of approximately104cells per sq.cm. in DMEM/F12 (50:50) supplemented with 5% FBS, 10ng/ml of bFGF, 10 ng/ml of EGF, and 100 uM dibuturyl cAMP. Typically DAN cells are used within 2-6 passages.
Gene Delivery using Nanoparticles:
Twenty four hours before siRNA transfection, ~20,000 dopaminergic neuronal (DAN) cells were seeded onto 6-well plates in OPTI-MEM containing 4% FBS with no antibiotics to give 30 to 50% confluence at the time of transfections. The siRNA was reconstituted in DNase-RNase free water to a final concentration of 10 μM and mixed with 20 μl of GNR solutions. The final concentration of siRNA for in vitro transfection of dopaminergic neurons was 100 nM. The commercially available siRNA delivery agent, Lipofectamine (Invitrogen) was used as the positive control in our experiments.
RNA Extraction:
Cytoplasmic RNA is extracted from DAN cells 48 hours post- transfection using Trizol reagent (Invitrogen- Life Technologies, Carlsbad, CA). The amount of RNA is quantitated using a Nano-Drop ND-1000 spectrophotometer (Nano-Drop ™ Wilmington, DE) and isolated RNA is stored at –80oC until used.
Real Time Quantitative PCR (Q-PCR):
DARPP-32 Gene expression was quantitated 48 hours post- transfection using real time quantitative PCR using DARPP-32 specific primers using the Brilliant® SYBR® green QPCR master mix from Stratagene (Stratagene Inc, La Jolla, CA; Cat # 600548-51). RNA is reverse transcribed to cDNA using the reverse transcriptase kit from Promega (Promega Inc, Madison, WI; Cat # A3500). Relative gene expression of DARPP32 is calculated using the comparativeCT method 27. All data are controlledfor quantity of RNA input by performing measurements on an endogenousreference gene, b-actin. Briefly, the analysis is performed asfollows.For each sample, a difference inCT values ( CT) is calculated for each mRNA by taking the meanCT and subtracting the mean CT for the reference RNA (b-actin) measured on an aliquotfrom the same RT reaction. The
CT) is calculated for each mRNA by taking the meanCT and subtracting the mean CT for the reference RNA (b-actin) measured on an aliquotfrom the same RT reaction. The  CT for the sample of interest is then subtracted fromthe
CT for the sample of interest is then subtracted fromthe  CT for the appropriate control sample to generate a
CT for the appropriate control sample to generate a 
 CT.The mean of these
CT.The mean of these 
 CT measurementsis then used to calculate the levels in the targeted cytoplamic RNA relativeto the reference gene and normalized to the untreated control as follows:Relative levels or Transcript Accumulation Index = 2-
CT measurementsis then used to calculate the levels in the targeted cytoplamic RNA relativeto the reference gene and normalized to the untreated control as follows:Relative levels or Transcript Accumulation Index = 2-
 CT. This calculation assumes that all PCR reactions are workingwith 100% efficiency. All PCR efficiencies were found to be >95%; therefore,this assumption introduces minimal error into the calculations.
CT. This calculation assumes that all PCR reactions are workingwith 100% efficiency. All PCR efficiencies were found to be >95%; therefore,this assumption introduces minimal error into the calculations.
Cell Viability Assay to Evaluate Toxicity of Nanoparticles:
MTS cell proliferation assay measures the reduction of a tetrazolium component (MTS) into an insoluble formazan product by the mitochondria of viable cells. The MTS assay is a quantitative, sensitive detection of cell proliferation since it measures the growth rate of cells by virtue of a linear relationship between cell activity and absorbance. DAN (10,000 cells/ml/well) were incubated with 4, 20, 40 and 80 ug/ml concentrations of GNR-PEG, GNR-PEI, GNR-PDDAC, GNR-CIT for 24 and 72 hours respectively. At the end of the incubation period DAN cells were treated with the MTS reagent for approximately 3 hours, followed by addition of a detergent solution to lyse the cells and solubilize the colored crystals. The samples were read using an ELISA plate reader at a wavelength of 490 nm.
Results and Discussion:
PEI and PDDAC coating were performed using a layer-by-layer regime. A thin layer of anionic polymer poly(styrenesulfate) (PSS) was deposited on the CTAB layer to facilitate the further attachment of biocompatible cationic polymers (GNR-PSS). Many reports suggested that the synthetic parameters such as the concentration and the ionic strength of anionic polymer in layer-by-layer (LBL) preparations should be precisely controlled in order to obtain well-defined nanoparticles with high yield 28, 29, 30, 31, 32, 33. In our case, fast injection of excess PEI or PDDAC into concentrated GNR-PSS on vigorous stir plate were employed to give well-dispersed, stabilized and high positively charged nanoparticles.
Another formulation took advantage of the unique covalent bond of thiol-Au(0) by replacing CTAB with amine-poly(ethylene glycoside)-thiol. GNR-PEG was accomplished by ligand exchange process. PEGylated GNR was obtained after 24 hours of stirring the mixture in a glass container. GNR-CIT hybrid nanoparticles were prepared by crosslinking chitosan using glutaraldehyde. Low molecular weight chitosan in 1% acetic acid was used to form a neat protection layer to the GNR and thus reduce the toxicity originated from CTAB. The use of EDTA served as a buffer layer between two cationic moieties, CTAB surface and chitosan chain. The GNR-EDTA-chitosan mixture was stirred for 4 hours to homogeneously disperse the solution. All formulations were purified from excess chemicals by centrifugation and redispersion in HPLC-grade water. Figure 1 illustrates the different strategies of coating GNRs with cationic materials and are defined as GNR-PEI, GNR-PDDAC, GNR-PEG and GNR-CIT. Morphological images and uniformly polymer coating of all formulations were shown in Figure 2. The length and width of GNRs are estimated to be 50 nm and 15 nm, respectively. Note that the core-shell structure of GNR-CIT was clearly shown under high resolution TEM (Figure 2c).
Figure 1.Preparation of cationic GNR-PEI, GNR- PDDAC, GNR-PEG and GNR-CIT. Three different techniques have been used: (i) Layer-by-layer, deposition of negatively charged layer (PSS) was followed by another cationic polymers (PEI and PDDAC) . (ii) Ligand exchange, the original ligand (CTAB) was replaced by amine functionalized thiolated PEG (iii) Encapsulation, GNR was encapsulated in a chitosan matrix by crosslinking chitosan shell using glutaraldehyde.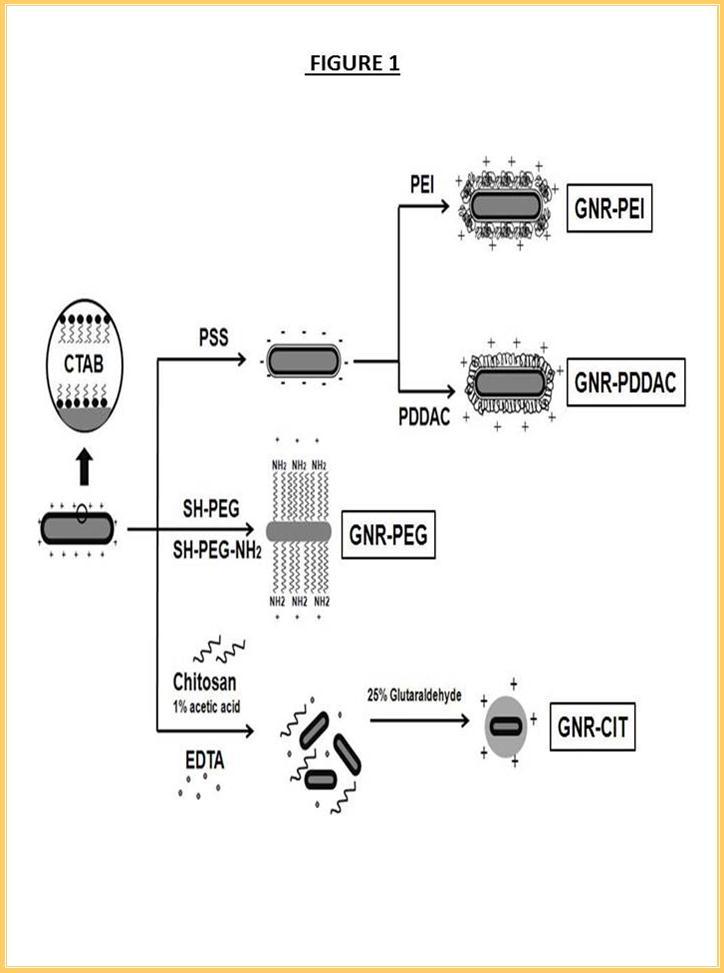
Figure 2.Transmission Electron Microscopy of (a) GNR-PEG, (b) GNR-PEI, (c) GNR-CIT, and (d) GNR-PDDAC. The shapes of GNR have been retained after the surface modifications.
After the surface modifications, red shifts in the longitudinal surface plasmon resonant peak, which can be accounted for the localize refractive index changes on the gold nanorod surfaces, were observed (Figure 3a and 3b). Zeta-potential of all coating formulations was recorded to assure that GNRs would electrostatically complex with negatively charged siRNA. It is also a good indicator to confirm the successful ligand exchange or polymer deposition. In Figure 3c, the highly positive charge of the bilayer CTAB was clearly reversed to negative after PSS deposition. The reversals of Zeta-potential were observed again after the growth of PDDAC or PEI layer. Ligand exchange from CTAB to NH2-PEG-SH and the formation of core-shell structure of GNR-CIT were also indicated from the reduction in Zeta-potential.
The Dynamic light scattering (DLS) technique is used as a valuable parameter to confer stability. The unstable and abrupt change of the hydrodynamic diameter of nanoparticles in biological medium greatly affects their function and behavior in vitro and in vivo. We studied the stability of GNRs with different surface modifications for 100 hours in different biologically relevant media such as water, phosphate buffered saline (PBS) and Dulbecco's Modified Eagle Medium (DMEM). As shown in Figure 3(d-f), all formulations showed stable dispersions in water. The nanoparticles are normally stabilized in a medium by their surface charges. When the nanoparticles are subjected to the high ionic strength biological fluids, self-assembled aggregation may occur due to the neutralization of surface charge by the ionic species. Short term stability of GNR-PDDAC, GNR-CIT and GNR-PEI were observed in PBS (Figure 3e). Their hydrodynamic diameters gradually increased after 24 hours. The presence of high content of amino acids and glucose in DMEM decreased the stability of the nanoparticles. Aggregation of GNR-CIT and GNR-PEI were found after 1 hour incubation in DMEM. GNR-PDDAC also aggregated after 24 hours of incubation in DMEM as shown in Figure 2f. It appears that the stability of the nanoparticle is highly correlated with its surface charge. GNR-PDDAC, with the highest Zeta–potential, exhibits more stability in media which can be attributed to a longer neutralization time by the ion containing liquid. Our results confirm reported literature that demonstrates a drop of Zeta–potential is observed in high ionic strength medium 32.
Our study revealed that GNR-PEG is extremely stable although it carries the least positive charge. As shown in Figure 3(d-f), the hydrodynamic diameter of GNR-PEG was maintained in all medium for 100 hours. The extra stability was conferred by the surface passivation with the PEG layer which has been known to prevent protein opsonization. It is worth mentioning that the aggregation and serum absorption of nanoparticles can disrupt its function and cause adverse effects such as degradation of passivation surfaces, the exposure of the unprotected core to the biological samples and cytotoxicity.
Figure 3.(a) Extinction spectra, (b) position of longitudinal absorption peaks, suggesting that the plasmonic properties of GNR is sensitive to the surface modications. (c) Zeta-potential shows the cationic surfaces of all samples. Hydrodynamic diameter of different surface coating GNRs (d) in water, (e) in PBS and (f) in cell culture medium. Among which, GNR-PEG exhibits excellent stability in all media.
Cytotoxicity of the various GNR formulation were tested using the MTS assay. Various concentrations of the different GNR formulations were incubated with DAN cells in DMEM with 10% fetal bovine serum (FBS). No cytotoxicity to DAN cells was observed after 24 or 72 hours of incubation even at the highest concentration of 80 µg/ml (Figure 4a and Figure 4b), indicating that aggregation of nanoparticles has no adverse effect DAN cells.
Figure 4.Cell viability of DAN cells at all formulations at (a) 24 hours and (b) 72 hours post treatment, indicating the minimal cytotoxicity of all GNR samples.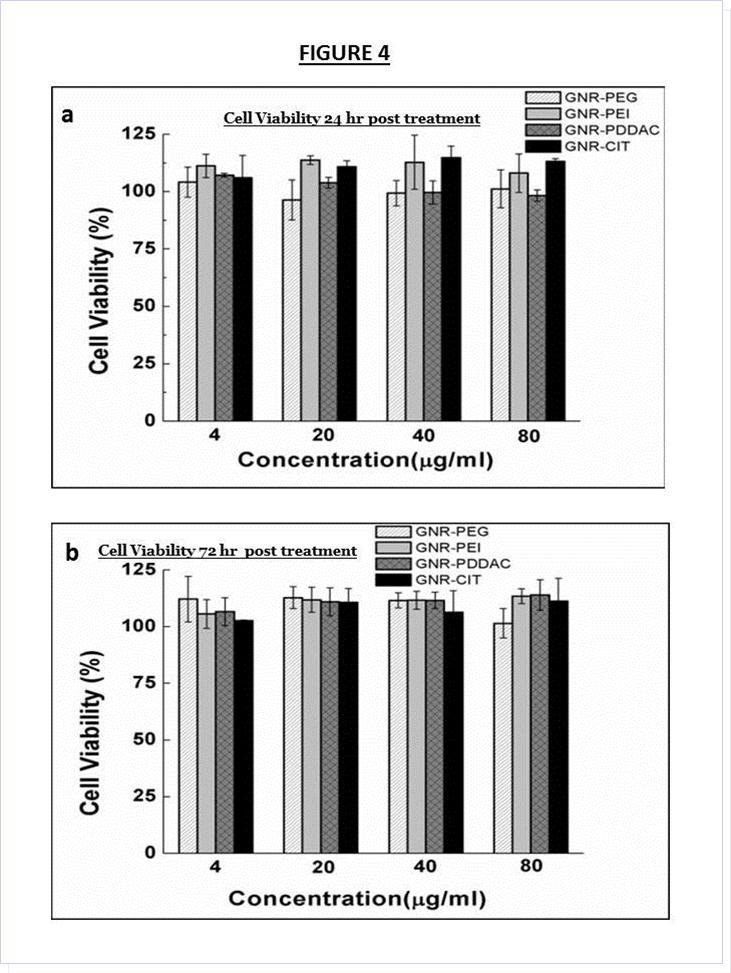
In vitro gene silencing studies were performed in DAN cells. DARPP-32 gene expression, was suppressed using the different GNR formulations complexed to DARPP-32 siRNA. All GNR formulations were incubated with the same concentration of DARPP-32 siRNA to form nanoplexes (GNR-PDDAC-siRNA, GNR-PEI-siRNA, GNR-PEG-siRNA, GNR-CIT-siRNA, Lipofectamine-siRNA) by electrostatic binding. The commercial available agent, Lipofectamine, was used as the positive control. DAN cells were treated with the nanoplexes and incubated for 48 hours. The efficiency of gene knockdowns were evaluated by using quantitative real-time (Q)-PCR technique 48 hours post-transfection. Figure 5 demonstrates that DAN cells treated with the various GNR siRNA nanoplexes, showed significant decrease in a DARPP32 gene expression. The percentage suppression in DARPP-32 gene expression was 38%, 40 %, 36%, and 39% for GNR-PDDAC-siRNA (p<0.01), GNR-PEI-siRNA (p<0.01), GNR-PEG-siRNA (p<0.01), and GNR-CTI-siRNA (p<0.01) respectively as compared to their respective controls that were not complexed to DARPP-32 siRNA. All the GNR nanoformulations showed gene silencing efficiencies comparable to the commercially available Lipofectamine reagent (42% gene suppression).
Figure 5.DARPP-32 gene silencing efficiency of the different nanoformulations of GNR ( i.e GNR- PDDAC, GNR-PEI, GNR-PEG and GNR-CIT respectively) and DARPP32siRNA complexes as compared to the transfection efficiency of Lipofectamine- DARPP-32 siRNA nanoplex in DAN cells.. DAN (1 x 105cells/ml) were treated in vitro with GNR-DARPP-32siRNA nanoplexes, and the commercially available transfection reagent Lipofectamine (Invitrogen) for 48 hr. RNA was extracted, reverse transcribed, cDNA amplified and the DARPP-32 gene expression was determined 48h hr post transfection by real time quantitative PCR. Relative expression of mRNA species was calculated using the comparativeCT method. Data are the mean ± SD of 3 separate experiments done in triplicate. Our results show a ~40% suppression in DARPP-32 gene expression in DAN cells that were transfected with the GNR-DARPP-32 siRNA nanoplexes. No significant differences were observed in DARPP-32 gene silencing between the various GNR nanoformulations and the transfection efficiency was similar to that of the commercially available transfection reagent lipofectamine.
Due to the strong plasmonic scattering of GNR, dark field microscopy was used to study the cellular uptake of the GNR-siRNA nanoplexes. Figure 6 shows representative dark field images of DAN cells without and with the treatment of GNR-PEG-siRNA. Intense light scattering from GNRs is clearly seen in Figure 6b when comparing with the untreated DAN cells image as shown in Figure 6a. This strong light scattering is associated with cellular uptake of GNR-PEG-siRNA nanoplexes. The cellular uptake of GNR-PEG-siRNA was also confirmed using confocal microscopy. 6-carboxyfluorescein, fluorophore (FAM)-labeled siRNA (siRNAFAM) was used to provide the fluorescence signal. As shown in Figure 6d, DAN cells treated with GNR-PEG-siRNAFAM nanoplexes showed stronger fluorescence signal than DAN cells treated with Lipo-siRNAFAM (Figure 6c).
Figure 6 .Dark-field image of (a) DAN cells alone and (b) GNR-PEG. The bright spots are associated with the scattering of GNR. Confocal image of (c) Lipo-siRNAFAM and (d) GNR-PEG-siRNAFAM. The green is pseudo-color representing the green fluorescence from FAM.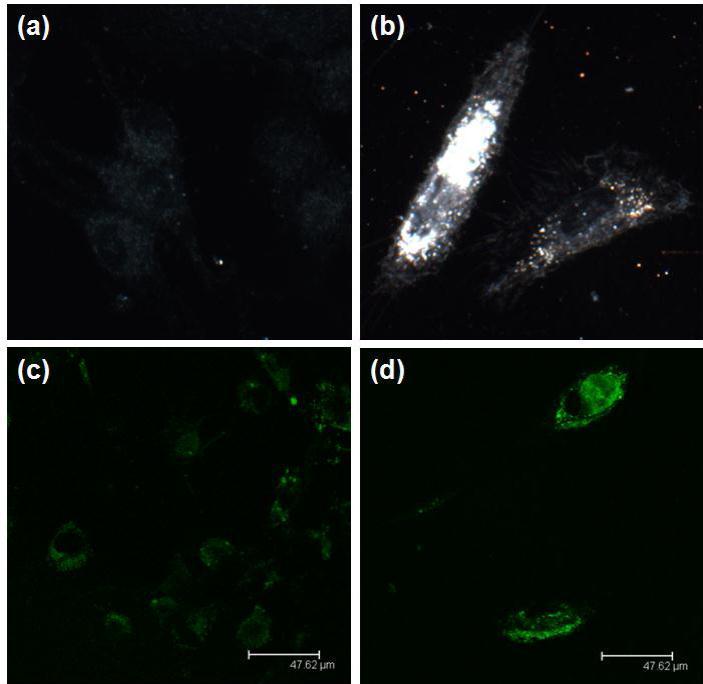
Conclusion:
Our data suggest that the gene silencing efficiency is similar for all GNR nanoformulations we tested and is comparable to the commercially available transfection agent lipofectamine. The GNR nanoformulations however, have an increased shelf life and stability as compared to lipofectamine. The accumulation of GNR inside cells has both positive and negative outcomes for biomedical applications. The positive outcome is that they can serve as multifunctional imaging and therapeutic agents for specific targeted cells. On the other hand, the negative outcome is the non-specific uptake of GNR by cells which cause an accumulation of GNR within the body and the physiological implications of which are unknown. The surface coating of GNR by PEG chains and other formulations as described in this report, is one an effective way to ensure their clearance from systemic circulation.
In summary, we have reported different coatings of GNRs for gene therapy. All coatings were confirmed by characterization techniques. Our prepared gene delivery nanoplatforms manifest a high biocompatibility and all of them are stable in biological medium. The suppression of DARPP-32 gene with all the four GNR –siRNA nanoformulations (GNR-PDDAC-siRNA, GNR-PEI-siRNA, GNR-PEG-siRNA, GNR-CIT-siRNA,) have comparable transfection efficiency with the commercially available transfection agent, Lipofectamine. In addition to efficient gene targeting, our nanoplatforms are capable of utilizing multimodal imaging. Further development and optimization of this technology could help stabilize other plasmonic nanostructures and could enable sensitive detection and specific gene targeting both in vitro and in vivo.
Acknowledgements
This study was supported by grants from the National Institute of Health NIDA-1R21DA030108-01 (S. Mahajan), K01DA024577 (J. Reynolds) and Royal Thai government (A. Kopwitthaya).
References
- 1.Guo P. (2004) RNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapy. , J Nanosci Nanotechnol 5(12), 1964-82.
- 2.Salata O. (2004) Applications of nanoparticles in biology and medicine. , Journal of Nanobiotechnology 2, Paper 3.
- 3.A K Salem, P C Searson, K W Leong. (2003) Multifunctional nanorods for gene delivery. , Nature Materials 2, 668-71.
- 4.Singh N, Agrawal A, AKL Leung, Sharp P A, Bhatia S N. (2010) . , Effect of Nanoparticle Conjugation on Gene Silencing by RNA Interference. Journal of the American Chemical Society;132: 8241-3.
- 5.Davis M E, Zuckerman J E, CHJ Choi, Seligson D, Tolcher A. (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature;464: 1067-70.
- 6.Tokatlian T, Segura T. (2010) siRNA applications in nanomedicine. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology;2: 305-15.
- 9.Ding H, K T Yong, Roy I, H E Pudavar, W C Law. (2007) Gold nanorods coated with multilayer polyelectrolyte as contrast agents for multimodal imaging. , The Journal of Physical Chemistry C; 111, 12552.
- 10.Dorfs D, Krahne R, Falqui A, Manna L, Giannini C.. et al.(2011) In Comprehensive Nanoscienceand Technology;David,LA.,Gregory,D.S.,Gary,P.W.,Eds.; Academic Press: Amsterdam,2011 219.
- 11.A de Fougerolles, Vornlocher H P, Maraganore J, Lieberman J. (2007) Interfering with disease: a progress report on siRNA-based therapeutics.Nat Rev DrugDiscov;6(6):. 443-53.
- 12.Aigner A. (2006) Delivery systems for the direct application of siRNAs to induce RNA interference (RNAi) in vivo. , J Biomed Biotechnol 4, 71659.
- 13.Higuchi Y, Kawakami S.Hashida M.(2010).Strategies for invivo delivery of siRNAs:recentprogress.BioDrugs.;24(3):. 195-205.
- 14.Raviña M, Paolicelli P, Seijo B.Sanchez A.(2010). Knocking down gene expression with dendritic vectors. , Mini Rev Med 10(1), 73-86.
- 15.Buxton D B. (2009) Nanomedicine for the management of lung and blood diseases. , Nanomedicine 4(3), 331-9.
- 16.H de Martimprey, Vauthier C, Malvy C, Couvreur P. (2009) Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA.EurJPharmBiopharm;71(3):. 490-504.
- 17.Fougerolles de.AR.(2008). Delivery vehicles for small interfering RNA in vivo. , Hum Gene Ther; 19(2), 125-32.
- 18.Toub N, Malvy C, Fattal E.Couvreur P.(2006). Innovative nanotechnologies for the delivery of oligonucleotides and siRNA. , Biomed Pharmacother; 60(9), 607-20.
- 19.Kopwitthaya A, Yong K-T, Hu R, Roy I, Ding H.et al.(2010). Biocompatible PEGylated gold nanorods as colored contrast agents for targeted in vivo cancer applications Nanotechnology;21:. 315101.
- 20.Bonoiu A C, Mahajan S D, Ding H, Roy I, Yong K-T. (2009) Nanotechnology approach for drug addiction therapy: Gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proceedings of the National Academy of Sciences; 106(14), 5546-50.
- 21.Bonoiu A, Mahajan S D, Ye L, Kumar R, Ding H. (2009) MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. , Brain Res; 1282, 142-55.
- 22.Mahajan S D, Aalinkeel R, Reynolds J L, Nair B, Sykes D E. (2012) Suppression of MMP-9 expression in brain microvascular endothelial cells (BMVEC) using a gold nanorod (GNR)-siRNA nanoplex. , Immunol Invest; 41(4), 337-55.
- 23.Law W C, Yong K T, Baev A, Prasad P N. (2011) Sensitivity improved surface plasmon resonance biosensor for cancer biomarker detection based on plasmonic enhancement. , ACS Nano; 5(6), 4858-64.
- 24.Zhu J, Yong K T, Roy I, Hu R.Ding H et al.(2010). Additive controlled synthesis of gold nanorods (GNRs) for two-photon luminescence imaging of cancer cells. , Nanotechnology; 21(28), 285106.
- 25.Song W-J, Du J-Z, Sun T-M, Zhang P-Z.Wang J.(2010). Gold Nanoparticles Capped with Polyethyleneimine for Enhanced siRNA Delivery. , Small; 6, 239-46.
- 26.Guo S, Huang Y, Jiang Q, Sun Y, Deng L.(2010).Enhanced Gene Delivery and siRNA Silencing by Gold Nanoparticles Coated with Charge-Reversal Polyelectrolyte. , ACS Nano; 4, 5505-11.
- 27.S A Bustin. (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. , J Mol Endocrinol; 1, 23-39.
- 28.Podesta J E, Al-Jamal K T, Herrero M A, Tian B. (2009) Antitumor Activity and Prolonged Survival by Carbon-Nanotube-Mediated Therapeutic siRNA Silencing in a Human Lung Xenograft Model. , Small; 5, 1176-85.
- 30.Wu J, Lizarzaburu M E, Kurth M J, Liu L, Wege H. (2001) Cationic Lipid Polymerization as a Novel Approach for Constructing New DNA Delivery Agents. , Bioconjugate Chemistry; 12, 251-7.
- 31.BCC Pessela, Betancor L, Lopez-Gallego F, Torres R, Dellamora-Ortiz G M. (2005) Increasing the binding strength of proteins to PEI coated supports by immobilizing at high ionic strength. , Enzyme and Microbial Technology; 37, 295-9.
Cited by (1)
- 1.Greener Megan R., Storr Sarah J., 2022, Exploring the Role of DARPP-32 in Addiction: A Review of the Current Limitations of Addiction Treatment Pathways and the Role of DARPP-32 to Improve Them, NeuroSci, 3(3), 494, 10.3390/neurosci3030035
