Abstract
This study aims at investigating the antipyretic activity of different solvent fractions of the root bark of Rutideaparviflora(Rubiaceae). This plant is used ethno-botanically by the people of Ethiope East-West Local Government Area of Delta State, Nigeria to treat various ailments such as inflammation, fever and pain. This necessitated this research to validate its local use, due to the scanty literature and information present about this plant. It has also shown some anti-cancer and anti-inflammatory activity in previous researches. The present study is a randomized control study. Acetic acid induced writhing was employed for analgesic testing. Acetic acid was used to induce writhing in Wistar rats which were divided into fourteen (14) groups. The groups were administered extracts and fractions of the plant (200 mg/kg and 400 mg/kg). The animals were observed for number of writing movements and the percentage writhing was calculated. Baker’s yeast induced pyrexia was employed for the antipyretic testing. The animal groups were administered extracts and fractions of the plant (200 mg/kg and 400 mg/kg), with Paracetamol as the standard drug (100 mg/kg) and Normal saline (control) for both experiments. The body temperature of the rats was measured rectally over a period of five (5) hours. All values of P<0.05 were taken as significant. The organic extract, aqueous extract and various fractions (n-hexane, ethyl-acetate, n-butanol and aqueous) produced significant inhibition of writhing responses and pyrexia in a dose dependent manner and time dependent manner respectively. The aqueous extract at a dose of 400mg/kg showed the greatest reduction in writhing, 91.58% compared to the standard drug (paracetamol) which may suggest that the fraction possesses better efficacy than paracetamol as an analgesic. The observed activities could be attributed to these bioactive compounds: Palmatine, Urs-12-ene-24-oic-3-oxo-methyl ester and Gallic acid contained in R. parviflora.
Author Contributions
Academic Editor: Fatma Mohammed Mady, Department of Pharmaceutics, Minia University, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Johnson-Ajinwo Okiemute Rosa, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Rubiaceae consist of various flowering plants called the madder family, bedstraw family or coffee family. The Rubiaceae family has 630 genera and almost 13000 species found worldwide in tropical and warm regions 1.
Many Rubiaceae family plants exhibit antimalarial, antimicrobial, anti-hypertension, anti-diabetic, antioxidant and anti-inflammatory activities. Bioactive compounds such as anthraquinones, alkaloids, indole and terpenoids have been isolated from these plants1.
Two important genera of Rubiaceae with demonstrated anti-inflammatory activities include Borreria and spermacoce. The documented bioactivities of their isolated compounds include anti-inflammatory, anti-tumor anti-microbial, larvicidal, anti-oxidant, gastrointestinal, anti-ulcer and hepato-protective effects 2. Recently, Preliminary in vivo investigations of Borreriaverticillata Linn (Rubiaceae) for analgesic and anti-inflammatory effects carried out indicated that the plant possessed significant (P>0.001) analgesic and anti-inflammatory activities at a dose range of 200 to 1000 mg/kg p.o/i.p in all models used 3.
Another related specie; Nauclealatifolia Smith (Rubiaceae) a small tree, native to Africa and used in traditional medicine by several indigenous communities for the treatment of fever, malaria, pain, epilepsy and anxiety has been found to induce hypothermia with significant antipyretic effects in mice. Also significant antinociceptive activity was recorded in all analgesia animal models used4.
Recently, Nauclealatifolia was investigated for its anti-pyretic, anti-nociceptive and anti-inflammatory activities in two animal models. The researchers’ documented significant outcomes in all the activities evaluated in a dose-dependent manner1.
There are scanty reports on the activities of R. parviflora. One study has documented the anti-cancer activity of this plant; R. parviflora 5. The evaluation of the cytotoxic activities of the compounds isolated from the R. parviflora plant demonstrated palmatine as the most potent bioactive compound. The anti-cancer activities of palmatine have been reported from several studies6,7,8. Further investigations of the cytotoxic activities of palmatine in apoptosis assays demonstrated that the compound was cytotoxic to ovarian cancer cells. 5
R. parviflora is used by the indigenous people of Ethiope East/West Local Government in Delta State, Nigeria. Based on its ethno-medicinal use by the indigenous people in the treatment of inflammation alongside its associated fever and pain, this research became necessary to validate the ethno-medicinal use of this plant for analgesic and antipyretic effects beneficial in the treatment of pain and fever.
Materials and Method
Materials
Collection, Identification and Authentication
R. parviflora (root bark) was sourced from a bio reserve in Nigeria. The plant was authenticated by a botanist, Mr. Alfred O. Ozioko (INTERCEDD) with expert advice offered by Prof. J.F Bamidele of the Department of Plant Biology and Biotechnology, University of Benin, Nigeria. The voucher specimen numbers of the plant was; Rutideaparviflora INTERCEDD/1588.
Equipment and Instruments
Electronic weighing balance (model WT6002A), Maceration jars, Thermostat bath (HH-6; Techmel and Techmel, USA), Lypholiser (Harvest right scientific freeze dryer), Beakers, Glass funnels, Measuring cylinders, Conical flask, Rotary evaporator (R-205), Desiccator, Spatula, Crucibles, Filter papers, Syringes and Digital thermometers.
Reagents
Methanol, Dichloromethane, n-hexane, Ethyl acetate and n-butanol of Analytical grade (Sigma-Aldrich). Distilled water was obtained from the Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Port Harcourt.
Methods
Extraction of the Plant Materials
The plant materials were extracted according to the American National Cancer Institute (NCI) method of extraction9. Typically, 200g of the pulverized plant material was macerated in a 1: 1 mixture of 500ml of dichloromethane and 500ml of methanol for 24 h. The ratio of plant material to solvent used was 1:5. This ratio was maintained for all weighed amount of plant materials used. The obtained solution containing the extracts was decanted off and 500ml of methanol was added to the residue and allowed to stand for another 24 h. The solution of the extract was collected by filtration and 1 liter of deionized water was added to the residue. The aqueous extract was collected after 24 h of maceration. The methanol extraction was combined with the 1:1 dichloromethane and methanol extraction to yield the organic extract. This extraction solution was evaporated to dryness on a rotary evaporator at a temperature of 40 ̊C. The obtained dry extracts were further dried in a desiccator to remove any trace of solvent. The aqueous extraction was dried using a lyophilizer to obtain a solid sample.
The percentage yields of the crude extracts of the plant calculated as follows
Percentage yield =
Sequential Fractionation of the Plant
The organic extracts of the plant (about 22g) with the potent activity was subsequently partitioned based on increasing polarity in n-hexane, ethyl-acetate, and n-butanol consecutively. The extract was first dissolved in 90% methanol in water and partitioned with n-hexane three times (100ml x 3). The combined hexane extract was evaporated to yield the n-hexane fraction. The 90% methanol fraction was evaporated and the resulting residue was dissolved in water and partitioned with ethyl acetate three times (100ml x 3), which upon evaporation, the ethyl acetate fraction was obtained. Finally, partitioning in n-butanol (100ml x 3) was carried out. After separation, collection and evaporation of the solvents, the n-butanol and aqueous fractions was obtained. These four (4) fractions were investigated for anti-pyretic and analgesic activity.
Phytochemical Screening
Experimental Design
This study was designed in line with the ethically approved experimental protocols adopted by the department of Experimental Pharmacology and Toxicology, of the Faculty of Pharmaceutical Sciences, University of Port Harcourt. Healthy adult Wistar albino rats irrespective of sex, weighing between 170-200 grams were selected for the study. These animals were obtained from the animal house of the Department of Pharmacology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu, Nigeria. The animals were exposed to 12 hours of dark light cycle and kept under room temperature and humidity. After the animals were selected for study, they were separated in cages and were given food and water. The animals were allowed to acclimatize to laboratory conditions for 14 days prior to the experiments.
Antipyretic Activity
Baker’s Yeast Induced Hyperthermia in Rats
The method established by Tomazetti et al, 2005 12, with some modifications was employed in the antipyretic activity evaluation, with fever induced by Brewer’s yeast in rats. The basal rectal temperature of each rat was recorded at zero hour using clinical digital thermometer. Pyrexia was induced by subcutaneous injection of 15% w/v suspension of Brewer’s yeast in distilled water at a dose of 10 ml/kg body weight. In order to ensure uniform spreading of the suspension beneath the skin, the injection site was massaged. Immediately after yeast administration, food was withdrawn but access to water was still maintained. After 18 hours of Brewer’s yeast injection the rise in rectal temperature was recorded and only animals showing an increase in temperature of at least 0.6°C (or 1°F) were selected for the study. The mean increment recorded was 0.96°C after 18 h of administration. The animals were randomly divided into 14 groups, each group containing five rats. Group I received normal saline orally. Group II was given standard drug Paracetamol at the dose of 100 mg/kg per-oral.
The remaining Groups were Treated Orally as Follows;
Groups III and IV received organic extract of the plant (R. parviflora) at oral dose of 200 mg/kg and 400 mg/kg respectively.
Groups V and VI received aqueous extract at oral dose of 200 mg/kg and 400 mg/kg respectively.
Groups VII and VIII received n-hexane fraction at oral dose of 200 mg/kg and 400 mg/kg respectively.
Groups IX and X received ethyl acetate fraction at a dose of 200 mg/kg and 400 mg/kg po respectively.
Groups XI and XII received n-butanol fraction at a dose of 200 mg/kg and 400 mg/kg po respectively.
Groups XIII and XIV received aqueous fraction at oral dose of 200 mg/kg and 400 mg/kg respectively.
After the treatment, the temperature of all the rats in each group was recorded at 0, 1, 2, 3, 4 and 5 hours.
Analgesic Activity
Acetic Acid Induced Writhing Test
The method described by Koster et al, 1959 13, was used for the evaluation of analgesic activity in rats. The experimental animals were weighed and randomly divided into 14 groups consisting of 5 rats in each. Group I (control) received normal saline (10 ml/kg) orally. Group II (positive control) received standard drug Paracetamol at oral dose of 100 mg/kg. Remaining groups were treated orally as follows:
Groups III and IV received organic extract at doses of 200 and 400 mg/kg respectively.
Groups V and VI received aqueous extracts at doses of 200 and 400 mg/kg respectively.
Groups VII and VIII received n-Hexane fractions at doses of 200 and 400 mg/kg respectively.
Groups IX and X received ethyl acetate fractions at doses of 200 and 400 mg/kg respectively.
Groups XI and XII received n-Butanol fractions at doses of 200 and 400 mg/kg respectively.
Groups XIII and XIV received aqueous fractions at doses of 200 and 400mg/kg respectively.
All treatments were administered orally. 45 minutes after administration of standard drug and test samples, each mouse was injected with 0.7% acetic acid at the dose of 10 ml/kg body weight intraperitoneally. The number of writhing responses manifested by each mouse was recorded for 30 minutes commencing just 5 minutes after acetic acid injection. The percentage analgesic activity was calculated as follows:
% inhibition of writhing = (wc-wt/wcX100)
Where W is number of writhing, Wc is control, and Wt is test.
Statistical Analysis
All values were expressed as the mean ± standard error of the mean (SEM) and the results were analyzed statistically by one-way analysis of variance (ANOVA) for analgesic activity and multivariate analysis of variance (MANOVA) for antipyretic effect through time followed by Dunnett’s post hoc multiple comparison test by using SPSS ver. 16. For MANOVA, Levene’s test of equality errors of variance was performed. P < 0.05 was considered to be statistically significant.
Results
Extraction
Weight of pulverized powder = 1300g
Percentage yield = 
Fractionation
Weight of Organic Extract used for fractioning = 22g
Percentage yield of fraction =
The results in Table 1, showed the percentage yield of the extracts and the fractions. It could be seen that the root bark of R. parviflora yielded less than 2% of the organic extract. This is not unexpected as roots tend to yield far less extracts than leaves or aerial parts of plants.
Table 1. Yield and Percentage Yield of Extracts and Fractions| EXTRACTS/FRACTIONS | YIELD (g) | PERCENTAGE YIELD (%) |
| Organic Extract(Methanol/Dichloromethane) | 24 | 1.85 |
| Aqueous Extract | 3.9 | 0.3 |
| N-Hexane Fraction | 2.1 | 9.55 |
| Ethyl Acetate Fraction | 3.3 | 15 |
| N-Butanol Fraction | 1.7 | 7.73 |
| Aqueous Fraction | 1.15 | 5.23 |
The phytochemical screening results are contained in Table 2. The findings include the presence of alkaloids, flavonoids, tannins, saponins and triterpenoids. There is documented evidence that plants with a combination of secondary metabolites such as alkaloids, flavonoids and saponins possess significant analgesic activities 14, 15. Similarly, alkaloids, steroids, tannins and terpenoids are predominant inhibitors of Prostaglandin synthases; suggestive of anti-pyretic activities 16,17.
Table 2. Result for Phytochemical Screening 11| 1 | ALKALOIDS TEST | |
| (a) | Meyers test | +ve |
| (b) | Dragendorff test | +ve |
| (c) | Hagers test | +ve |
| 2 | FLAVONOIDS TEST | |
| (a) | Shinoda test | +ve |
| (b) | Sodium hydroxide test | +ve |
| 3 | TANNINS | |
| (a) | Ferric chloride test | +ve |
| 4 | CARBOHYDRATE TEST | |
| (a) | Molish test | +ve |
| (b) | Fehling’s test | +ve |
| 5 | SAPONIN TEST | |
| (a) | Frothing test | +ve |
| (b) | Emulsion test | +ve |
| 6 | PHLOBATANNINS | |
| (a) | Hydrochloride acid test | -ve |
| 7 | GLYCOSIDE TEST | |
| (a) | Keller killiani test | +ve |
| (b) | Kedde test | +ve |
| 8 | TRITERPENOIDS | |
| (a) | Leiberman-buchard test | -ve |
| (b) | Salkwoski test | +ve |
Palmatine a quaternary protoberberine alkaloid that was previously isolated from Rhizomacoptidis, an important medicinal plant commonly used in the Traditional Chinese Medicine, TCM 18,has previously being isolated from R. parviflora. Also the presence of Urs-12-ene-24-oic-3-oxo-methyl ester; a pentacyclic triterpeniod and Gallic acid have been documented in R. parviflora5.
Antipyretic Test Result
The results of the anti-pyretic evaluation of the extracts and fractions of R. parviflora are presented in Table 3 and Figure 1 above. The results showed a rapid onset of activity by the 400 mg/kg doses of the extracts and fractions that were comparable to the standard drug PCM. The aqueous extract yielded very significant activity (P>0.05) than PCM; thus displaying superior anti-pyretic activity against PCM at 1 H. The extracts and fractions demonstrated significant effect on rectal temperature with significant reduction of temperature over a period of 3-5 H.
Figure 1.A Line Graph Showing the Antipyretic Effect of the Extracts of the Root Bark of Rutidea parviflora (Rubiaceae) and Its Fractions on Yeast Induced Pyrexia
| Variables | Basal Temp. ( ̊C) | Rectal Temperature ( ̊C) | |||||
| 0hr (after 18hr) | 1hr | 2hr | 3hr | 4hr | 5hr | ||
| Control | 37.8 ± 0.30a | 39.05 ± 0.55b | 38.50 ± 1.3b | 37.95 ± 1.65a | 37.00 ± 0.5a | 36.75 ± 1.48a | 36.15 ± 1.25a |
| Paracetamol (100mg/kg) | 37.75 ± 0.30a | 38.90 ± 0.10a | 37.65 ± 0.75a | 36.60 ± 0.50a | 35.30 ± 0.00ac | 35.0 ± 0.00ac | 35.70 ± 0.65a |
| Organic Crude (200mg/kg) | 38.50 ± 0.40b | 39.20 ± 0.00b | 38.10 ± 0.30b | 37.20 ± 0.50a | 36.10 ± 0.20a | 35.9 ± 0.45ac | 35.70 ± 0.20a |
| Organic Crude (400mg/kg) | 35.05 ± 0.25a | 37.50 ± 0.00a | 37.05 ± 0.07a | 36.95 ± 0.25ac | 36.1 ± 0.25ac | 36.3 ± 0.65a | 36.45 ± 0.35a |
| Aqueous Crude (200mg/kg) | 36.55 ± 0.25a | 37.75 ± 0.15ac | 37.05 ± 0.45a | 36.60 ± 0.50a | 35.95 ± 1.05c | 35.90 ± 0.8ac | 35.80 ± 0.10a |
| Aqueous Crude (400mg/kg) | 35.90 ± 0.20a | 36.75 ± 0.45ac | 36.10 ± 0.40ac | 36.10 ± 0.5ac | 35.75 ± 1.15c | 35.75 ± 0.15ac | 35.65 ± 0.55a |
| n-Hexane Fraction (200kg/mg) | 37.60 ± 0.1a | 38.15 ± 0.25a | 38.05 ± 0.15ac | 37.55 ± 0.15ac | 36.9 ± 0.1ac | 36.6 ± 0.05a | 35.95 ± 0.15a |
| n-Hexane Fraction (400kg/mg) | 37.1 ± 0.6a | 37.7 ± 0.6a | 36.5 ± 0.4b | 35.7 ± 0.3a | 35.7 ± 0.05ac | 35.55 ± 0.25a | 35.4 ± 0.3a |
| Ethyl Acetate Fraction (200mg/kg) | 37.65 ± 0.05a | 38.45 ± 0.25a | 37.35 ± 0.15a | 37.2 ± 0.3ac | 36.8 ± 0.1c | 36.7 ± 1.2c | 36.10 ± 1.2a |
| Ethyl Acetate Fraction (400mg/kg) | 36.5 ± 0.7a | 37.05 ± 0.75 | 36.45 ± 0.85a | 36.30 ± 0.3a | 35.65 ± 0.85ac | 35.55 ± 0.65a | 35.55 ± 0.75b |
| n-Butanol Fraction (200mg/kg) | 38.05 ± 0.55b | 39.10 ± 0.10b | 37.8 ± 0.00a | 37.40 ± 1.6a | 36.65 ± 0.45a | 36.55 ± 0.25c | 36.45 ± 0.55b |
| n-Butanol Fraction (400mg/kg) | 36.95 ± 0.35a | 37.6 ± 0.05a | 37.15 ± 0.25a | 36.88 ± 0.55ac | 36.75 ± 0.25ac | 36.50 ± 0.6a | 36.45 ± 0.35b |
| Aqueous Fraction (200mg/kg) | 37.95 ± 0.55a | 38.6 ± 0.15a | 38.20 ± 0.10b | 37.90 ± 0.4a | 37.40 ± 0.9a | 37.00 ± 0.3b | 36.95 ± 0.15b |
| Aqueous Fraction (400mg/kg) | 37.35 ±0.15a | 38.25 ± 0.05a | 36.75 ± 1.35a | 36.60 ± 0.9ac | 36.00 ± 0.95c | 35.90 ± 1.8ac | 35.45 ± 1.45a |
Further evaluation of the anti-pyretic activities was the extracts/fractions were carried out by calculating the percentage reduction of pyrexia as seen in Figure 2 below.
Figure 2.A Bar Chart Showing the % Reduction of Pyrexia in Rats after administration of the Extracts of the Root Bark of Rutidea parviflora (Rubiaceae) and Its Fractions on Yeast Induced Pyrexia
A break-down of the percentage reduction of pyrexia in the rats displayed in Figure 2 was in consonance with the observations made in Table 1 and Figure 1 previously; that the extracts and fractions had their maximum % reduction between 3-5 H; an indication that the herbal extracts and fractions had long lasting activity. The results at 1-2 H of the 400 mg/kg doses of the extracts/fractions and PCM showed that the activities were comparable. Also, the aqueous extract had a very significant activity (P>0.05) at 1 H, which surpassed that of PCM and the other extracts/fractions. But at the 3rd and 4th hour respectively PCM significantly inhibited pyrexia with the maximum % reduction being observed at the 4th hour of the experiment.
The analgesic effects of the extracts/fractions of R. parviflora were investigated and the results obtained are presented in Table 4, Figure 3 and Figure 4 respectively.
Table 4. Analgesic Effect of Extracts from Root Bark of Rutidea parviflora (Rubiaceae) and Its Fractions on Acetic Acid Induced Writhing Test.| Variables | Acetic Acid Induced Writhing Test (Analgesic Test) | % writhing |
| Control | 94.50 ± 0.85a | 0 |
| Paracetamol (PCM) (100mg/kg) | 15.05 ± 0.44b | 84.21 |
| Organic Extract (200mg/kg) | 77.03 ± 0.48a | 17.89 |
| Organic Extract (400mg/kg) | 12.89 ± 0.24b | 86.32 |
| Aqueous Extract (200mg/kg) | 52.75 ± 0.84ac | 44.21 |
| Aqueous Extract (400mg/kg) | 7.96 ± 0.83b | 91.58 |
| n-Hexane Fraction (200kg/mg) | 18.09 ± 0.29b | 81.05 |
| n-Hexane Fraction (400kg/mg) | 11.75 ± 0.41b | 87.37 |
| Ethyl Acetate Fraction (200mg/kg) | 16.07 ± 0.41b | 83.16 |
| Ethyl Acetate Fraction (400mg/kg) | 13.09 ± 0.71b | 85.26 |
| n-Butanol Fraction (200mg/kg) | 48.91 ± 0.28c | 48.42 |
| n-Butanol Fraction (400mg/kg) | 8.97 ± 0.41b | 90.53 |
| Aqueous Fraction (200mg/kg) | 33.98 ± 0.82c | 64.21 |
| Aqueous Fraction (400mg/kg) | 11.06 ± 0.35b | 88.42 |
Figure 3.Line Graph for Analgesic Effects of Extracts of the Root Bark of Rutidea parviflora (Rubiaceae) and its Fractions on Acetic Acid Induced Writhing Test
Figure 4.Bar Chart for Analgesic Effects of Extracts of the Root Bark of Rutidea parviflora (Rubiaceae) and Its Fractions on Acetic Acid Induced Writhing Test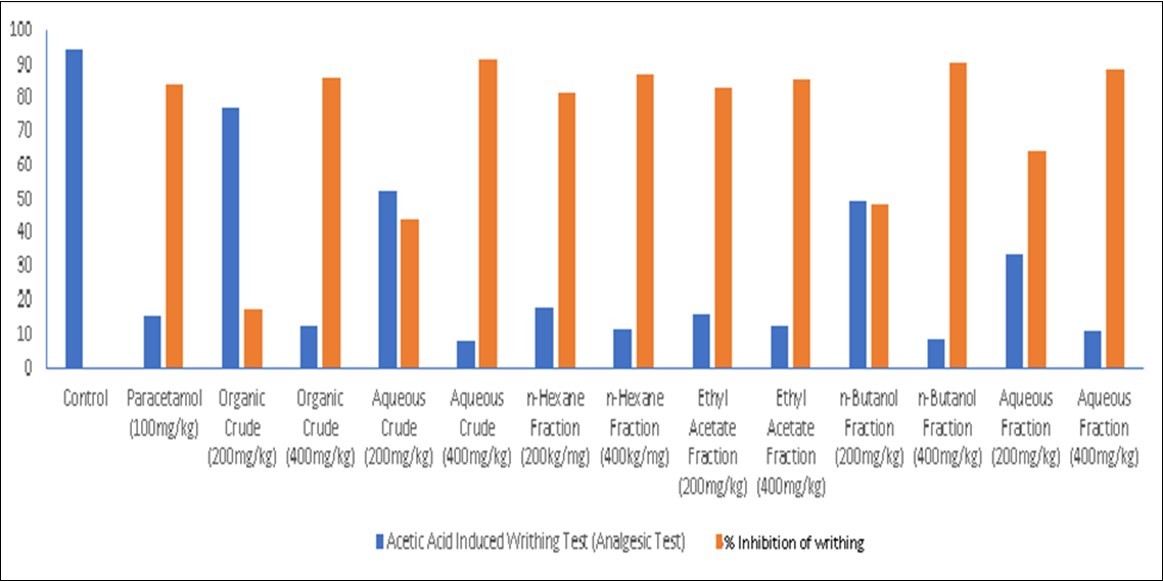
Analgesic Test Result
The results of the analgesic experiments carried out are displayed in Table 4, and Figure 3 and Figure 4. There are strong indications that R. parviflora possess significant analgesic activities. This is evident from the dose-dependent and time-dependent significant activities recorded for the extracts and fractions. The analgesic result again validates the anti-pyretic activities of the extracts and fractions. It could be observed that the 400 mg/kg doses were comparable or superior to PCM. These results clearly support the indigenous use of this plant in the treatment of inflammations and fevers. Previous studies have linked the presence of alkaloids, flavonoids, tannins, terpenoids, saponins and steroids with good anti-pyretic activities. Saponins are known to inhibit the enzymes involved in the formation of pyrexia, while flavonoids hinders the synthesis of PG2 by inhibiting tumour necrosis factor –α responsible for the induction of fever. 16,17
Discussion
The ethno-medicinal use of plants for the treatment of various ailments including microbial infections, neurological conditions, inflammation, pain and fever is common in Nigeria. The result of the phytochemical screening suggests that flavonoids and saponins are present which may explain this plant’s antipyretic and analgesic activities. Other secondary metabolites present in the plant include alkaloids, tannins and glycosides. Numerous studies conducted on medicinal plants have associated the presence of metabolites such as flavonoids and alkaloids to anti-inflammatory, analgesic and antipyretic properties 16. Flavonoids are proposed to reduce arachidonic acid release through inhibition of neutrophils degranulation 19 leading to the suppression of prostaglandins and leukotrienes responsible for inflammation, pain, and fever.
The extracts and different fractions from the root bark of R. parviflora(Rubiaceae) were assessed for analgesic activity against acetic acid induced writhing which is an indicator of visceral pain. The specific pain activity generated by intra-peritoneal injection of acetic acid is indicated with contraction of abdominal muscle associated with the stretching of hind limbs, elongation of the abdomen and other similar movements which are assumed to be mediated by local peritoneal receptor 20. The acetic acid triggered writhing is an economic, common and easy method for testing analgesic drugs 21. Acetic acid administration is responsible for the release of endogenous substances which excite the nerve endings, thereby causing the pain 22. Numerous researches have revealed the buildup of higher levels of prostaglandins (such as PGE2 and PGF2α) 23, lipoxygenase products (such as leukotrienes) 24, and resident mast cells 25, in fluids treated with pain inducing acetic acid and increase in pain sensation through capillary permeability 26.
The main impact of prostaglandins in producing pain response is mostly due to interaction with endogenous substances such as histamine, bradykinin, and substance P which further stimulate the sensitization of pain receptors to these mediators 27. NSAIDs relieve pain by inhibiting peripherally, the production of endogenous substances such as prostaglandins, thromboxane, and other inflammatory mediators by acting on cyclooxygenase enzymes (COX 1 and COX 2). Any substance that reduces the number of abdominal constrictions induced by acetic acid can be considered to have analgesic activity.
The extracts and fractions of R. parviflora significantly reduced the number of writhing in dose dependent mode. The extracts and fractions at the dose of 200 mg/kg and 400 mg/kg produced significant reductions in the number of writhing (P < 0.05) produced by acetic acid in rats when compared to the control group.
The aqueous extract at a dose of 400 mg/kg showed the greatest reduction in writhing, 91.58% (P< 0.05) compared to the standard drug (paracetamol) which showed a reduction in writhing of 84.21% and the other samples. However, the organic fraction at a dose of 200 mg/kg showed an almost insignificant reduction in writhing of 17.89% but produced significantly better analgesia at a dose of 400mg/kg which was 86.32% (P< 0.05). This strongly suggests that the plant under study possesses good peripheral analgesic property, possibly elicited by a similar mechanism of action to the standard drug (paracetamol) through the inhibition of prostaglandins, thereby decreasing inflammation along with its associated pain and fever.
The extracts and different fractions from the root bark of R. parviflora (Rubiaceae) were also assessed for antipyretic activity against yeast induced fever which is an indicator of pathogenic fever. The yeast induced fever is an economic, common and easy method for testing antipyretic drugs 12. The proteins present in yeast induce fever by stimulation of inflammation 28. Furthermore, the production of endogenous pyrogens such as pro-inflammatory cytokines (interleukin IL, interferons IFN and tumor necrosis factors TNF)29 and prostaglandins (PGE2 and PGI2) 30 are responsible for increasing the temperature of the body by acting on the hypothalamus in the brain 31.
Antipyretics such as paracetamol employed for use in management of pyrexia have several mechanisms of action such as reducing prostaglandins levels by acting on cyclooxygenase (COX) enzymes, enhancing hypothalamus thermo-regulatory activities and managing anti-inflammatory signals such as inflammatory cells and molecules 32.
The subcutaneous injection of yeast markedly increased the rectal temperature and the mean increment recorded was 0.96°C after 18 h of administration. The different treatment extracts/fractions of the plant and paracetamol lowered the rectal temperature in time dependent manner 33. Several researches carried out by numerous researchers have shown that medicinal plants showing anti-inflammatory activity may also possess antipyretic and analgesic activities 34 as the mechanism of action elicited for the suppression of inflammation, fever, and pain can be linked to the inhibition of mediators of inflammation.
Previous studies by our research team have obtained palmatine and urs-12-ene-24-oic acid, 3-oxo-methyl ester from R. parviflora. While gallic acid was identified from the GCMS determination of the organic extract of R. parviflora. Palmatine an anti-cancer agent; has been documented to possess anti-inflammatory and anti-pyretic activities amongst other activities35. Previous researchers have documented the anti-asthma, anti-arthritic, anti-inflammatory, anti-microbial and diuretic activities of Urs-12-ene-24-oic acid, 3-oxo-methyl ester36. Recently, the anti-pyretic and analgesic activities of gallic acid was reported from an investigation of the anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice. LC–MS/MS analyses revealed the presence of anthocyanin, catechin, gallic acid, resveratrol, quercetin, flavone, flavonols and epicatechin as the active constituents of the plant 37.
Conclusion
The extracts and different fractions from the root bark of R. parviflora (Rubiaceae) displayed significant (P < 0.05) analgesic and antipyretic properties of equal potency when compared to the standard drug paracetamol. The aqueous extract at a dose of 400 mg/kg showed the greatest reduction in writhing, 91.58% compared to the standard drug (paracetamol) which may suggest that it possesses better efficacy than paracetamol as an analgesic.
The precise mechanisms involved in the production of the antipyretic and analgesic activities of the plant may be caused by the presence of the palmatine, urs-12-ene-24-oic acid, 3-oxo-methyl ester and gallic acid in the root-bark of R. parviflora (Rubiaceae).
References
- 1.Simplice D Karou, TchadjoboTchacondo Denise P Iiboudo, Simpore Jacques. (2011) Sub-Saharan Rubiaceae: A review of their traditional uses, phytochemistry and biological activities”,Pakistan. , Journal of Biological Sciences 14, 149-169.
- 2.L M Conserva, Ferreira J C Jr. (2012) (Rubiaceae): A review of their ethno medicinal properties, chemical constituents and biological activities”,PharmacognRev. 6(11), 46-55.
- 3.H S Abdullahi-Gero, Ahmed A, A U Zezi, I M Hussaini. (2014) Preliminary evaluation of ethanol leaf extract of Borreria verticillata Linn (Rubiaceae) for analgesic and anti-inflammatory effects. , J. Med. Plants Res 8(20), 736-747.
- 4.G S Taïwe, E N Bum, Talla E, Dimo T, Weiss N et al. (2011) Antipyretic and antinociceptive effects of Nauclea latifolia root decoction and possible mechanisms of action. , Pharmaceutical biology 49(1), 15-25.
- 5.R Johnson Ajinwo O, Richardson A, Li W. (2019) Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins (Basel). 11, 237.
- 6.Vennerstrom J, Klayman D. (1988) Protoberberine alkaloids as antimalarials. , J Med Chem 31(6), 1084.
- 7.Wu J, Xiao Q, Zhang N, Xue C, Leung A et al.Photodynamic action of palmatine hydrochloride on colon adenocarcinoma HT-29 cells. Photodiagnosis Photodyn Ther. 1572(16), 30056-2016.
- 8.Wu J, Xiao Q, Zhang N, Xue C, Leung A et al. (2016) Palmatine hydrochloride mediated photodynamic inactivation of breast cancer MCF-7 cells: Effectiveness and mechanism of action. Photodiagnosis Photodyn Ther. 15-133.
- 9.Thomas G. (2010) High Throughput Extraction of Plant, Marine and Fungal Specimens for Preservation of Biologically Active Molecules. , Molecules 15, 4526.
- 11.O R Johnson-Ajinwo, C E Udofia, A O Nwanosike. (2020) Phytochemical Screening and. In Vivo Anti-Inflammatory Activities of Anti-Cancer Plant: Rutidea parviflora (Rubiaceae). Clinical Oncology & Research 3(10), 1-7.
- 12.Tomazetti J, D S Ávila, A P Ferreira. (2005) Baker yeast-induced fever in young rats: characterization and validation of an animal model for antipyretics screening,”. , Journal of Neuroscience Methods 147(1), 29-35.
- 13.Koster R, Anderson M, Beer E J De. (1959) acid-induced analgesic screening,”Federation Proceedings. 18, 412-417.
- 14.O A Salawu, B A Chindo, A Y Tijani, Adzu B Analgesic. (2008) anti - inflammatory, anti-pyretic and anti-plasmodial effect of the methanolic extract of Crossopteryx. , febrifuga.Journal of Medicinal Plant Research 2(8), 213-218.
- 15.M Y Sani, M A, H A Yaro, B M Sani, Amoley A et al. (2013) Phytochemical screening and evaluation of analgesic and anti - inflammatory activities of the methanol leaf extract ofCissus polyantha. , Journal of Medical Sciences 13, 824-828.
- 16.S A Kumar, Venkatarathanamma V, N V Saibabu, S K Ram. (2015) Antipyretic activity of Annona plant leaves on brewer’s yeast induced febrile rats.Asian. , Journal of Pharmaceutical and Clinical Research 8(3), 210-212.
- 17.J K Kamau, P M Nthiga, V C Safari, S M Njagi, J K Mwonjoria et al.(2016).Antipyretic properties of methanolic stem bark extract ofAcaciahockiiDe wild andKigeliaAfricana(Lam). Benth in Wistar rats.Journal of Pharmacognosy and Natural Products 2(3), 1-6.
- 18.Chen P, Lu L, Y, Li Y.(2012).Determination of protoberberine alkaloids inRhizomaCoptidisby. , ERETIC H NMR method.J Pharm Biomed Anal 23(60), 44.
- 19.Tordera M, M L Ferrandiz, M J Alcaraz. (1994) Influence of anti-inflammatory flavonoids on degranulation and arachidonic acid release in rat neutrophils,” Zeitschrift für Naturforschung Section C;. , Journal of Biosciences,49(3-4): 235-240.
- 20.G A Bentley, S H Newton, Starr J. (1983) Studies on the anti-nociceptive action of α-agonist drugs and their interactions with opioid mechanisms,”. , British Journal of Pharmacology 79(1), 125-134.
- 21.H O Collier, L C Dinneen, C A Johnson, Schneider C. (1968) The abdominal constriction response and its suppression by analgesic drugs in the mouse,”. , British Journal of Pharmacology and Chemotherapy 32(2), 295-310.
- 22.G F Pavao-de-Souza, A C Zarpelon, G C Tedeschi. (2012) Acetic acid- and phenyl-p-benzoquinone-induced overt pain-like behavior depends on spinal activation of MAP kinases. PI3K and microglia in mice,” Pharmacology Biochemistry and Behavior 101(3), 320-328.
- 23.Deraedt R, Jouquey S, Delevallée F, Flahaut M. (1980) of prostaglandins E and F in an algogenic reaction and its inhibition,”EuropeanJournal of Pharmacology. 61(1), 17-24.
- 24.J D Levine, Lau W, Kwiat G, E J Goetzl. (1984) B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes,”Science. 225(4663), 743-745.
- 25.R A, M L Vale, S M Thomazzi.(2000).Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice,”European. , Journal of Pharmacology 387(1), 111-118.
- 26.Amico-Roxas M, Caruso A, Trombadore S, Scifo R, Scapagnini U. (1984) antinociceptive effects in rodents,”ArchivesInternationalesdePharmacodynamieetdeTherapie. 272(1), 103-117.
- 27.Davies P, P J Bailey, M, A W Ford-Hutchinson. (1984) role of arachidonic acid oxygenation products in pain and inflammation,”Annual Review of Immunology. 2(1), 335-357.
- 28.Pasin J S M, Ferreira A P O, Saraiva A L L.(2010).Diacerein decreases TNF-α and IL-1β levels in peritoneal fluid and prevents Baker's yeast-induced fever in young rats,”Inflammation. , Research 59(3), 189-196.
- 29.G N Luheshi. (1998) Cytokines and fever: mechanisms and sites of action,”Annals of the New York. , Academy of Sciences 856, 83-89.
- 30.W L Veale, K E Cooper, Q J Pittman. (1977) Role of prostaglandins in fever and temperature regulation, in The Prostaglandins,P.Ramwell,Springer,NewYork,USA.145-167.
- 31.C B Saper, C D Breder. (1994) neurologic basis of fever,The New. , England Journal of Medicine 330(26), 1880-1886.
- 32.D M Aronoff, E G Neilson.(2001).Antipyretics: mechanisms of action and clinical use in fever suppression,”The. , American Journal of Medicine 111(4), 304-315.
- 33.K S Nirmal, S M Abdur-Rahman, A. (2016) Analgesic and Antipyretic. Activities of Methanol Extract and Its Fraction from the Root ofSchoenoplectusgrossus”,Evidence-Based Complementary and AlternativeMedicine, Volume 2016, Article ID 3820704 .
- 34.Afsar T, M R Khan, Razak S, Ullah S, Mirza B. (2015) Antipyretic, anti-inflammatory and analgesic activity ofAcaciahydaspicaR. Parker and its phytochemical analysis,”BMC. , Complementary and Alternative Medicine 15, 136.
- 35.Küpeli E, Kosar M, Yesilada E, Baser K H C, Baser C. (2002) A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species.Life Sciences;. 72, 645-657.


