Abstract
Despite the generally accepted benefits of exercise on sleep there remains limited research on potential differential effects by exercise type. The purpose of the present study was to examine short-term and chronic effects of aerobic and resistance exercise on various sleep quality parameters, as well as sleep duration. Generally healthy, previously sedentary young (27±3 years) men completed a 16-week aerobic and 16-week resistance exercise program in random order separated by a minimum of 6 weeks with no formal exercise. Each exercise program consisted of three supervised exercise sessions per week. Quality and duration of sleep was determined with a multi-sensor device that was worn prior to, during week 1 and week 16 of each exercise program. A total of 8 participants provided valid data on time spent awake after sleep onset, sleep latency, total sleep time, sleep efficiency and time spent in bed for both exercise programs. During week 1, aerobic exercise was associated with a significant decline in sleep latency (-6.5±6.8 min) and time in bed (-39.2±42.2 min) while resistance exercise was associated with a decline in time spent awake after sleep onset (-21.6±16.7 min) and increased sleep efficiency (4.3±4.8 %). Effects were no longer significant after 16 weeks of exercise. These results indicate that aerobic and resistance exercise have beneficial effects on quality of sleep, particularly in the short-term, but the specific exercise-induced changes vary by exercise type.
Author Contributions
Academic Editor: Daniel Oshi, Department of Community Health and Psychiatry, The University of the West Indies, Mona, Kingston, Jamaica
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Clemens Drenowatz
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Sleep and physical activity (PA) are important modifiable behaviors that contribute to overall health 1, 2. Both, sleep and PA are associated with metabolism, endocrine and immune function as well as appetite regulation, which affect various chronic diseases, including, cardiovascular disease, diabetes and obesity 3, 4. PA and sleep are further associated with functional capacity, life satisfaction and mental health 5, 6, 7. Nevertheless, sleep difficulties and low levels of PA are common in many industrialized countries 2, 8. Particularly young adults are vulnerable to insufficient sleep and low sleep quality, which may, in part, be attributed to transitions in life (e.g., new job, starting a family, timing of social engagement). Even though sleep disturbances can be treated pharmacologically in the short term there remain concerns regarding rapid tolerance and dependency in the long term 9. Further, sleeping pills have been associated with increased risk for depression, cancer and mortality 10. PA, on the other hand, has been shown to have many positive side-effects and may be considered as a low-cost treatment addressing sleep problems 7. Due to the low demands of PA in our daily lives 11, exercise, which represents planned and structured PA 12, becomes an increasingly important contributor to total PA. Further, it has been suggested that exercise is a more potent contributor to quality of sleep than habitual and household PA 13.
Beneficial effects of exercise on quality and duration of sleep have been attributed to the role of sleep in energy conservation, body tissue recovery and temperature regulation 14. In addition, it has been suggested that exercise positively affects other health behaviors, such as consistent sleep-wake schedules, and reduced caffeine and alcohol intake, which are associated with quality of sleep 15, 16. Accordingly, exercise participation has been associated with a reduction in the severity of various sleep disorders, including insomnia, obstructive sleep apnea and periodic limb movements during sleep 17, 18, 19. Reported effects of exercise on sleep in the general population without major sleep problems, however, have generally been small and varied in robustness 8. Equivocal findings may, at least partially, be attributed to differential effects of exercise by study population (age, gender, fitness level) as well as training protocol (i.e. exercise type, duration, intensity) 15, 20. Most exercise interventions relied on aerobic exercise providing limited evidence on the role of other exercise routines such as resistance exercise on objectively determined sleep measures. Given the different acute physiological responses and adaptations to resistance exercise, compared to aerobic exercise, there may be differences in the effects on sleep. The purpose of the present pilot study, therefore, was to examine short-term and chronic effects of aerobic and resistance exercise on objectively measured duration and efficiency of nocturnal sleep in young, previously sedentary men. A better understanding of the potential differential effects of aerobic and resistance exercise could provide viable information for the development of behavioral interventions targeting sleep problems.
Methods
A total of 12 men between 19 and 35 years of age were recruited via, flyers and e-mail list-servs. Phone interviews were conducted in order to determine eligibility of interested participants. In order to be included in the study, participants needed to be free of any major acute or chronic diseases and have not been engaging in structured exercise for the previous 6 months. Additional inclusion criteria were no major lifestyle changes and less than 3% change in body weight during the previous three months (based on self-report). Further, participants needed to be able to complete the prescribed aerobic and resistance exercise program. Besides the exercise intervention participants were told to maintain their current dietary and lifestyle habits. All participants signed written informed consent and the study was approved by the Institutional Review Board of the University of South Carolina.
Exercise Program
Participants completed two 16-week exercise programs consisting of aerobic and resistance exercise in random order, separated by at least 6 weeks with no structured exercise. All exercise sessions were supervised and performed in an indoor training facility under constant environmental conditions. Prior to the beginning of each exercise program participants completed one orientation session to introduce them to the exercise protocol and determine the appropriate intensity. Each exercise program consisted of 3 supervised exercise sessions per week that were completed between Monday and Friday. Participants were allowed to consume water during the exercise sessions. The time of day for the exercise sessions were held consistent for each participant throughout each 16-week exercise block with the majority of participants completing their exercise sessions in the late afternoon or early evening hours (between 4:00pm and 7:00pm). Previous research also did not show any evidence for a disruption of sleep following exercise performed prior to sleep time, as opposed to shortly after waking, in young men 21, 22.
Aerobic exercise sessions were performed on a treadmill (True Fitness Technology, St. Louis, MO) and consisted of a 10-minute warm-up at 3.5 mph at 0% incline followed by a 45-minute continuous exercise bout at an intensity between 60% and 75% of the participant’s maximum heart rate, which was determined via a graded exercise test prior to starting the exercise intervention. Exercise sessions ended with a 5-minute cool-down at a self-determined walking pace at 0% incline. Heart rate was monitored continuously throughout the exercise session (Polar RS 400, Polar Electro Inc., Lake Success, NY). Participants self-selected the speed and incline during the 45-minute exercise bout according to their fitness level and adjusted the intensity as their fitness increased in response to the exercise training. Laboratory staff verified the appropriate intensity every 5-10 minutes. The resistance workout consisted of 10 machine-based exercises targeting the whole body. Specifically, participants completed 3 sets with 8 to 12 repetitions on 5 upper body exercises (lat pull, bench press, biceps curl, triceps extension, shoulder press), 3 lower body exercises (leg press, leg curl, leg extension) and 2 core exercises (abdominal crunches, back extension). In order to adjust for neuromuscular adaptations, resistance on the individual exercises was increased when participants completed 3 sets of 12 repetitions on 2 consecutive exercise days. Including the same warm-up and cool-down as described with the aerobic exercise, resistance exercise sessions lasted on average 65±10 minutes.
Anthropometric Measurements
Body weight (kg) and height (cm) was measured after an overnight-fast at baseline (prior to each exercise program), following the first week of exercise and at the end of the exercise program with participants wearing surgical scrubs and in bare feet. Measurements were performed in duplicates to the nearest 0.1 kg and 0.1 cm using an electronic scale (Healthometer model 500 KL, McCook, IL, USA) and a wall-mounted stadiometer (Model S100, Ayrton Corp., Prior Lake, MN, USA). Body mass index (BMI, kg/m2) was calculated using the average of both measurements. In addition, fat mass and fat free mass were measured via dual x-ray absorptiometry (DXA; GE Healthcare Lunar model 8743, Waukesha, WI) before and at the end of each exercise program. Percent body fat (BF) was calculated (fat mass/body weight) at the respective time points.
Sleep Measures and Physical Activity
Participants wore a SenseWear Mini armband (BodyMedia Inc., Pittsburgh, PA) on the upper left arm for 1 week prior to each exercise program, during week 1 and week 16 of each exercise program. Using tri-axial accelerometry, galvanic skin response, heat flux, skin temperature and near body temperature the armband has been shown to provide accurate measures of physical activity and sleep 23, 24. Participants were asked to wear the armband for 24 h/day and only remove it during periods when it might get wet, such as taking a shower. In order to be included in the analyses participants needed to provide data for a minimum of 5 days (including at least 1 weekend day) with a minimum of 21 hours/day. SenseWear’s proprietary algorithm (version 8.0 professional) was used to determine nocturnal sleep/wake and lying down for each minute the device was worn. Using these minute-by-minute data, the following average daily measures were created for nights following days with supervised exercise and days without supervised exercise: 1) sleep onset, represented by the first of three consecutive minutes asleep with at least ten minutes spent lying down; 2) sleep latency, which was the time between sleep onset and lying down; 3) wake-after-sleep-onset (WASO), calculated as the total time spent awake (at least 2 consecutive minutes) after sleep onset until wake time; 4) sleep duration, representing time spent sleeping between onset and waking; 5) wake time, determined as the first of 90 consecutive minutes spent awake in the morning; and 6) sleep efficiency, calculated as sleep duration divided by the length of time in bed (i.e., onset to waking). In addition, the armband was used to estimate time spent in moderate-to-vigorous PA (MVPA > 3 METs).
Statistical Analysis
Descriptive characteristics were calculated. Upon confirmation of normal distribution via Kolmogorov-Smirnov tests independent sample t-tests were used to examine differences in baseline characteristics between participants starting with aerobic or resistance exercise. Short-term and chronic effects of exercise on various sleep measures were examined via dependent t-tests comparing baseline measures to week 1 and week 16 of the respective exercise program, respectively. Due to the limited currently available information on sleep fluctuations during nights following exercise training and no training, sleep measures were examined separately for exercise days and non-exercise days. In addition, repeated measures ANCOVA, adjusting for exercise order, was used to explore potential differences between exercise and non-exercise days in relation to baseline measures. Given the variability in sleep behaviors between weekdays and weekend statistical analyses were conducted for the total week and for weekdays only. All statistical analyses were conducted using SPSS 22.0 (version 22.0; IBM Corp., Armonk, NY, USA) with a p-value of 0.05 and Bonferroni adjustment for multiple comparisons.
Results
A total of 8 (5 Caucasian, 3 Asian) participants provided valid PA and sleep data throughout both 16-week intervention periods. These participants completed 99.1±1.5% and 98.5±1.8% of the aerobic and resistance exercise sessions, respectively. Average armband wear-time during the respective measurement periods was 23.0±0.8 hours/day. Based on BMI, 2 participants were normal weight (19 kg/m2 ≤ BMI < 25 kg/m2), 5 participants were overweight (25 kg/m2 ≤ BMI < 30 kg/m2) and 1 participant was obese I (30 kg/m2≤ BMI < 35 kg/m2). There were no differences in descriptive characteristics at baseline, prior to either exercise program, between the 4 participants starting with aerobic and the 4 participants starting with resistance exercise (Table 1). Similarly, there were no differences in sleep measures prior to the aerobic and resistance exercise program . No significant change in body composition was observed throughout the entire training period but VO2peak increased significantly by 3.2±1.6 ml/kg/min with aerobic exercise (p<0.01), independent of exercise order.
Short-term effects of exercise on sleep. Aerobic and resistance exercise was associated with a 46.0±30.8 min/day and 34.1±22.4 min/day increase in time spent in MVPA from baseline to week 1 for aerobic and resistance exercise, respectively (p<0.01). Relative to baseline sleep measures aerobic exercise induced a significant decline in sleep latency (-6.5±6.8 min, p=0.03) and time in bed (-39.2±42.2 min, p=0.03) during week 1. Resistance exercise was associated with a decline in WASO (-21.6±16.7 min, p=0.01) and increase in sleep efficiency (4.3±4.8 %, p=0.04) from baseline to week 1. Neither exercise participation was associated with a change in sleep duration (p>0.11). Repeated measures analyses further indicated that differences in the sleep parameters between baseline and week 1 of either exercise program were more pronounced following non-exercise days rather than following exercise days (Table 2). Nevertheless, there was no difference between exercise and non-exercise days during week 1 of either exercise program. Comparing sleep measures between aerobic and resistance training showed a longer sleep duration following resistance exercise at week 1 (p=0.03).
Table 1. Baseline characteristics prior to either exercise program for the total sample and separately for those starting with aerobic and resistance exercise. Values are Mean±SD.| Total sample | Start with Aerobic Exercise | Start with Resistance Exercise | T-value | p-value | |
| Age (years) | 27.0 ± 3.0 | 28.2 ± 0.9 | 25.8 ± 4.1 | 1.097 | 0.315 |
| Height (cm) | 177.0 ± 7.7 | 172.7 ± 7.1 | 181.3 ± 6.1 | -1.825 | 0.118 |
| Weight (kg) | 88.4 ± 15.8 | 82.9 ± 7.8 | 94.0 ± 21.0 | -1.032 | 0.342 |
| BMI (kg/m2) | 28.0 ± 3.1 | 27.7 ± 0.6 | 28.4 ± 4.7 | -0.138 | 0.894 |
| Fat Mass (kg) | 27.2 ± 9.8 | 25.2 ± 1.7 | 28.4 ± 4.7 | -0.737 | 0.489 |
| Lean Mass (kg) | 58.7 ± 7.9 | 55.9 ± 7.3 | 61.5 ± 8.5 | -0.921 | 0.393 |
| % Body Fat | 30.0 ± 6.3 | 30.2 ± 2.5 | 29.9 ± 9.2 | -0.204 | 0.845 |
| MVPA (min/day) | 77.8 ± 19.7 | 86.2 ± 25.8 | 69.4 ± 9.9 | 1.1485 | 0.188 |
| VO2peak (ml/kg/min) | 35.8 ± 5.1 | 35.8 ± 5.0 | 35.8 ± 6.1 | 0.020 | 0.985 |
| Aerobic Exercise | Resistance Exercise | |||||
| Baseline | Week 1 | Baseline | Week 1 | |||
| EX days | No EX days | EX days | No EX days | |||
| WASO (min) | 81.3 ± 43.7 | 64.6 ± 59.2 | 65.2 ± 55.6 | 79.0 ± 46.2 | 66.5 ± 68.6 | 51.2 ± 40.1 ** |
| Latency (min) | 22.0 ± 8.0 | 20.9 ± 12.9 | 11.0 ± 4.8 * | 16.2 ± 10.0 | 21.1 ± 13.7 | 18.3 ± 9.4 |
| Total sleep (min) | 362.7 ± 40.4 | 340.9 ± 67.0 | 339.5 ± 56.7 | 334.9 ± 78.6 | 385.5 ± 75.6 | 344.4 ± 71.5 |
| Time in Bed (min) | 489.5 ± 43.4 | 455.1 ± 62.8 | 441.5 ± 45.6 | 451.7 ± 73.6 | 495.9 ± 52.1 | 432.8 ± 67.4 |
| Sleep efficiency | 74.2 ± 9.1 | 75.8 ± 12.9 | 77.2 ± 14.6 | 74.1 ± 12.5 | 77.9 ± 14.0 | 78.9 ± 11.7 |
Chronic effects of exercise on sleep. Time spent in MVPA remained significantly higher after 16 weeks of exercise, compared to baseline with either exercise program (∆MVPAaerobic= 25.3±12.2 min/day, ∆MVPAresistance= 38.5±23.8 min/day, p<0.01). Sleep measures following 16 weeks of either aerobic or resistance exercise, however, did not differ significantly from baseline sleep measures (Figure 1). Even though not significant, there was a trend towards a shorter latency with aerobic exercise (p=0.06), while resistance exercise showed a tendency for an increase in latency compared to baseline (p=0.07). Repeated measures analyses further revealed a significantly longer sleep duration following days of resistance exercise compared to non-exercise days at week 16 (mean difference 20.7±16.9 min, p=0.03). Results remained essentially unchanged when only weeknights (Sunday through Thursday) were considered for the analyses.
Figure 1.Change in sleep measures after 16 weeks of aerobic (a) and resistance (b) exercise. Values are mean with S.E.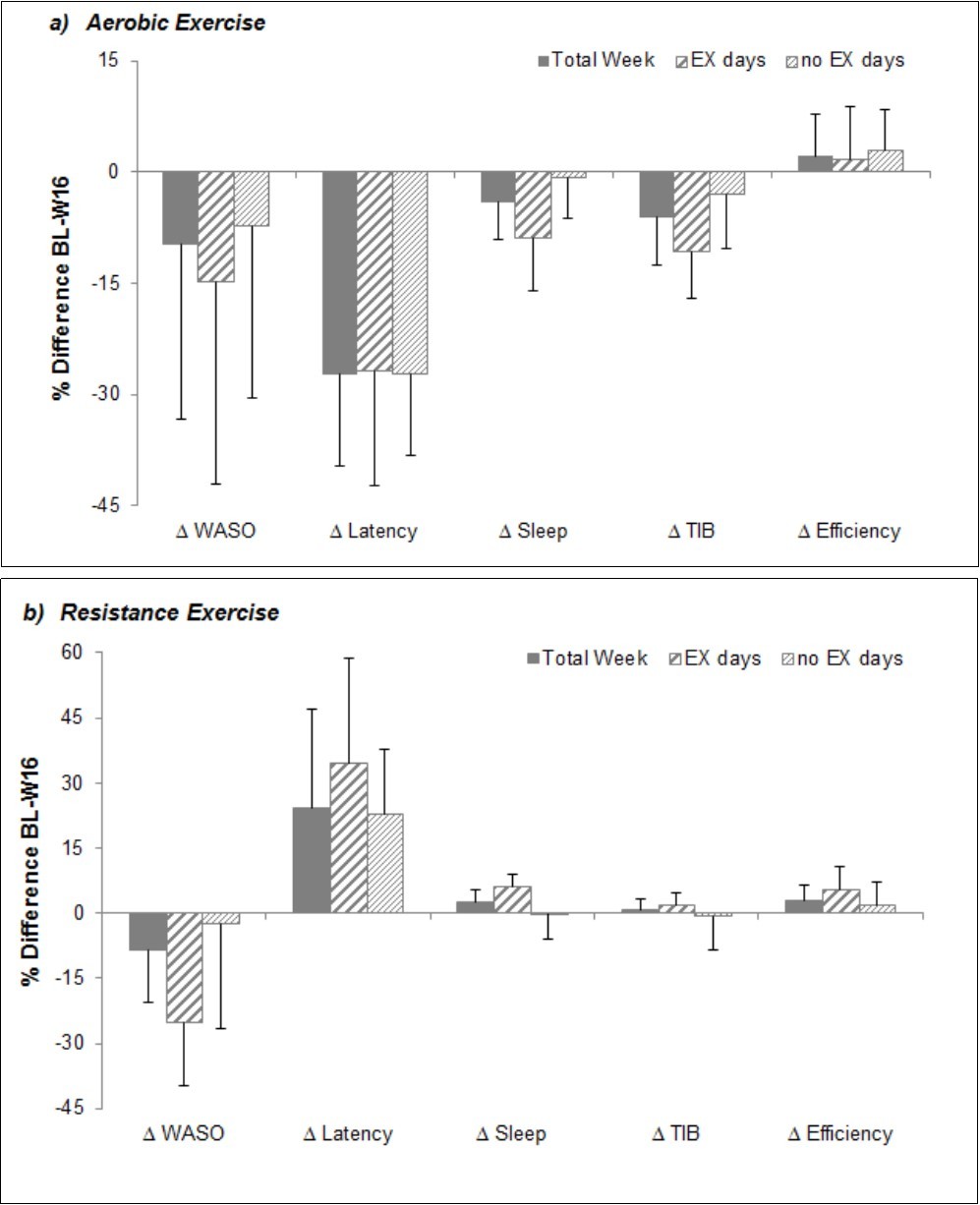
Discussion
Results of the present study indicate that participation in aerobic and resistance exercise induces positive changes in sleep measures, particularly in the short-term, but the nature of the improvement differed by exercise type. Aerobic exercise was associated with a reduction in latency and time in bed, while resistance exercise was associated with a reduction in WASO and increase in sleep efficiency. Additionally, resistance exercise was associated with longer sleep duration. A recent review on the association between PA and sleep in young adults also concluded that any kind of exercise has a positive impact on sleep 25. Specifically, previous research indicated a greater ease of falling asleep and deepness of sleep with exercise 9.
Interestingly, improvements in sleep quality were particularly pronounced on non-exercise days with limited effects of exercise on sleep during the night following the exercise bout. While it may be surprising that improvements in sleep quality were more pronounced following non-exercise days, it should be considered that beneficial effects of exercise on sleep could be masked by other exercise-induced physiological and mental effects, particularly in participants not accustomed to an exercise program. High intensity exercise, for example, has been shown to increase WASO 26 and the proportion of light sleep during nights after the exercise session in sedentary young adults 27. Further, even trained participants displayed a disruption of sleep with increased wakefulness following a marathon 28. These acute effects of exercise on sleep may no longer be present or most likely are less pronounced during nights further removed from the exercise bout. Excess post-exercise oxygen consumption (EPOC), for example, has been shown to last for up to 48 hours but values were significantly lower on the second day 29, 30. In absence of other exercise-induced changes (i.e. hormonal alterations, body temperature) the elevated metabolism on the 2nd day following an exercise bout, nevertheless, may contribute to increased sleep quality as higher energy expenditure has been suggested to improve sleep efficiency, particularly in young adults 31.
The present study further showed that exercise-induced effects on objective sleep measures were less pronounced with either exercise type after prolonged participation in an exercise program. Previous research also indicated that routinely practiced exercise does not alter sleep quality in generally healthy adults 15, 32. An increase in total sleep time, which occurred in response to resistance exercise in the present study, however, has been shown with other exercise interventions as well 8, 15. The greater effect of resistance exercise on sleep duration may be attributed to greater progression in absolute exercise intensity (i.e. increased weight on machines) with this type of training, while changes in aerobic exercise intensity (i.e. speed or incline) were minimal. Further, Gerber et al. 33 showed that participation in vigorous exercise is associated with longer sleep time. The lack of adjustment in aerobic exercise intensity despite an increase in cardiorespiratory capacity in the present study may have resulted in an insufficient stimulus to affect sleep parameters in the long term.
In addition, it should be considered that participants were generally healthy and good sleepers, which would limit beneficial effects of exercise on sleep as it leaves little room for improvement. Most likely there exists a floor or ceiling effect, which makes it difficult to achieve significant exercise induced changes in sleep quality unless participants display more severe sleep impairments prior to the intervention 20, 34. Even sleeping pills induced only slightly better improvements in sleep compared to exercise in good sleepers 34. Limited effects of chronic exercise, however, could also be attributed to a commonly observed decline in habitual PA, particularly in response to aerobic exercise 35. Such a compensatory behavior change would limit the exercise-induced increase in PA and total daily energy expenditure, which could mitigate the effect of exercise on sleep quality. In fact, it has been suggested that total PA or total daily energy expenditure, rather than exercise per se affect sleep efficiency 31. Accordingly, Youngstedt et al. (2003) argue that sleep quality may be more enhanced following a day of high PA consisting of light to moderate intensity compared to a day with a single exercise bout but otherwise spent predominantly sedentary 20. Given that most adults engage in limited PA during their daily lives, exercise should, nevertheless, be considered a viable option to enhance sleep quality.
The importance of total time spent in moderate-to-vigorous PA may be attributed to the association between total daily energy expenditure and the need for energy conservation and tissue restoration during sleep 15. Specifically, a high catabolic activity in response to increased energy expenditure has been associated with elevated anabolic activity during recovery and enhanced sleep quality. Additionally, an increase in core body temperature in response to exercise or moderate-to-vigorous PA has been suggested to activate heat loss mechanisms that contribute to better sleep 1, 15. Neural fatigue along with changes in heart rate variability and endocrine function in response to exercise also have been suggested as moderators of exercise-induced alterations of sleep 8. Further, exercise has been associated with improved mood state, which is also associated with better sleep quality 36. Given the complex interaction of physiological and psychological aspects, a detailed discussion of specific mechanisms that explain the association between specific exercise types and sleep is beyond the scope of this manuscript 37. The complex interaction, however, emphasizes the need for additional research using various exercise regimens in different populations. A recent review further emphasized the need to determine the specific underlying mechanisms that explain the association between exercise and sleep 8.
Some limitations of the present study need to be considered when interpreting the findings. Along with the previously mentioned generally normal and healthy sleep in the participants, the small sample size may have contributed to the lack of significant findings. Further, it has been shown that men have generally smaller adjustments in sleep quality in response to chronic exercise compared to women 38. In addition, sleep measures were obtained via accelerometry, which does not provide any information on different sleep stages and quality of sleep. Accelerometry does, however, provide objective data on various sleep characteristics and can be applied in the participants’ home with minimal influence on the normal sleep pattern 31. In contrast to laboratory studies, participants in the present study self-selected their sleep and wake-times, which may have also contributed to smaller alterations in sleep quality. Exercise participation, however, may affect sleep habits, such as going to bed later or getting up earlier in order to make time for exercising, which could affect sleep measures such as time in bed or sleep latency. The high compliance with the exercise and measurement protocol should also be considered as a strength of the present study.
Conclusion
In summary, findings of the present study support the notion that exercise has beneficial effects on various sleep measures. Effects of exercise on specific sleep measures, however, differ by exercise type, with aerobic exercise facilitating falling asleep, while resistance exercise was associated with better sleep efficiency. In order to enhance our understanding of the association between exercise participation and sleep, more research is needed that helps in determining the optimal exercise type and dose (i.e. exercise intensity and duration) for various populations, including participants with sleep problems. As sleep is an important contributor to overall health and well-being such research not only provides viable information on the individual level; it could also help in reducing the economic burdeon associated with poor sleep.
References
- 1.Chennaoui M, Arnal P J, Sauvet F, Léger D. (2015) Sleep and exercise: a reciprocal issue? Sleep Med Rev. 20, 59-72.
- 2. (2010) World Health Organization Global recommendations on physical activity for health.Geneva,Switzerland:WHOPress.
- 4.Löllgen H. (2013) Importance and evidence of regular physical activity for prevention and treatment of diseases. , Dtsch Med Wochenschr 138(44), 2253-2259.
- 5.Pilcher J J, Ginter D R, Sadowsky B. (1997) Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. , J Psychosom Res 42(6), 583-596.
- 6.Zeitlhofer J, Schmeiser-Rieder A, Tribl G, Rosenberger A, Bolitschek J. (2000) Sleep and quality of life in the Austrian population. , Acta Neurol Scand 102(4), 249-257.
- 7. (2008) US Department of Health and Human Services. physical activity guidelines for Americans. http://www.health.gov/paguidelines/guidelines/. AccessedMay5,2017
- 8.M A Kredlow, M C Capozzoli, B A Hearon, A W Calkins, Otto M W. (2015) The effects of physical activity on sleep: a meta-analytic review. , J Behav Med 38(3), 427-449.
- 9.Buman M, King A. (2010) Exercise as a treatment to enhance sleep. , Am J Lifestyle Med.2010; 4(6), 500-514.
- 10.D F Kripke, R D Langer, L E Kline. (2012) Hypnotics' association with mortality or cancer: a matched cohort study. , BMJ Open 2(1), 000850.
- 11.T S Church, D M Thomas, Tudor-Locke C, P T Katzmarzyk, C P Earnest. (2011) Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 6(5), 19657.
- 12.C J, K E Powell, G M Christenson. (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 100, 126-131.
- 13.Youngstedt S, Freelove-Charton J. (2005) Exercise and sleep. In:Faulkner G,Taylor A,editors. Exercise,health and mental health. London:Routledge 159-189.
- 16.Oaten M, Cheng K. (2006) Longitudinal gains in self-regulation from regular physical exercise. , Br J Health Psychol.11(Pt4): 717-733.
- 17.A M Esteves, Mello M T de, Pradella-Hallinan M, Tufik S. (2009) Effect of acute and chronic physical exercise on patients with periodic leg movements. Med Sci Sports Exerc. 41(1), 237-242.
- 18.C E Kline, E P Crowley, G B Ewing, J B Burch, S N Blair. (2011) The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. , Sleep 4(12), 1631-1640.
- 19.G S Passos, Poyares D, M G Santana, C V D’Aurea, S D Youngstedt. (2011) Effects of moderate aerobic exercise training on chronic primary insomnia. , Sleep Med 12(10), 1018-1027.
- 20.S D Youngstedt, M L Perlis, P M O’Brien, C R Palmer, M T Smith. (2003) No association of sleep with total daily physical activity in normal sleepers. , Physiol Behav 78(3), 395-401.
- 21.P J O'Connor, M J Breus, S D Youngstedt. (1998) Exercise-induced increase in core temperature does not disrupt a behavioral measure of sleep. , Physiol Behav 64(3), 213-217.
- 22.S D Youngstedt, D F Kripke, J A Elliott. (1999) Is sleep disturbed by vigorous late-night exercise? Med Sci Sports Exerc. 31(6), 864-869.
- 23.Welk G J, McClain J J, Eisenmann J C, Wickel E E. (2007) Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity (Silver Spring). 15(4), 918-928.
- 24.B T Peterson, Chiao P, Pickering E, Freeman J, G K Zammit. (2012) Comparison of actigraphy and polysomnography to assess effects of zolpidem in a clinical research unit. , Sleep Med 13(4), 419-424.
- 25.Lang C, Kalak N, Brand S, Holsboer-Trachsler E, Pühse U et al. (2016) The relationship between physical activity and sleep from mid adolescence to early adulthood. A systematic review of methodological approaches and meta-analysis. Sleep Med Rev. 28, 28-41.
- 26.S D Youngstedt, P J O’Connor, R K Dishman. (1997) The effects of acute exercise on sleep: a quantitative synthesis. , Sleep 20(3), 203-214.
- 27.Wong S, Halaki M, Chow C. (2013) The effects of moderate to vigorous aerobic exercise on the sleep need of sedentary young adults. , J Sports Sci 31(4), 381-386.
- 28.Montgomery I, Trinder J, Paxton S, Fraser G, Meaney M et al. (1985) Sleep disruption following a marathon. , J Sports Med Phys Fitness.25(1-2): 69-74.
- 29.Speakman J R, Selman C. (2003) Physical activity and resting metabolic rate. Proc Nutr Soc 62(3), 621-634.
- 30.Wilmore J H, Stanforth P R, Hudspeth L A, Gagnon J, Daw E W. (1998) Alterations in resting metabolic rate as a consequence of 20 wk of endurance training:. , the HERITAGE Family Study. Am J Clin Nutr 68(1), 66-71.
- 31.M H Oudegeest-Sander, T H Eijsvogels, R J Verheggen, Poelkens F, M T Hopman. (2013) Impact of physical fitness and daily energy expenditure on sleep efficiency in young and older humans. , Gerontology 59(1), 8-16.
- 32.Maculano Esteves A, Ackel-D'Elia C, Tufik S, Mello M T De. (2014) Sleep patterns and acute physical exercise: the effects of gender, sleep disturbances, type and time of physical exercise. , J Sports Med Phys Fitness 54(6), 809-815.
- 33.Gerber M, Brand S, Herrmann C, Colledge F, Holsboer-Trachsler E et al. (2014) Increased objectively assessed vigorous-intensity exercise is associated with reduced stress, increased mental health and good objective and subjective sleep in young adults. , Physiol Behav 135, 17-24.
- 34.S D Youngstedt. (2003) Ceiling and floor effects in sleep research. , Sleep Med Rev 7(4), 351-365.
- 35.Drenowatz C, Grieve G L, DeMello M M. (2015) Change in energy expenditure and physical activity in response to aerobic and resistance exercise programs. , Springerplus 4, 798.
- 36.Uchida S, Shioda K, Morita Y, Kubota C, Ganeko M et al. (2012) Exercise effects on sleep physiology. , Front Neurol 3, 48.
